Simple retrograde cerebral perfusion is as good as complex antegrade cerebral perfusion for hemiarch replacement
Introduction
Successful resection of aneurysm of aortic arch was first described by Drs. DeBakey and Cooley in the late 1950s (1). Prior to this, they tried to reconstruct the aortic arch under hypothermia with shunting, but none of the patients survived. Thus, they utilized cardiopulmonary bypass with bicaval drainage and arterial return to the femoral artery and selective perfusion to the innominate and left common carotid artery while the aorta proximal and distal to the aortic arch was clamped. However, this technique was also associated with high morbidity and mortality, and discouraged surgeons from performing the aortic arch repair at the time. The involvement of the transverse aortic arch necessitates temporary arrest of cerebral circulation, which causes ischemic insults to the brain. In addition, the manipulation to the aortic arch and its vessels causes embolization of thrombus and/or atheromatous debris to the brain. Cerebral complications often lead to poor outcomes after aortic repairs. Incidence of deaths in patients with permanent neurologic deficit (PND) is reported as high as 47% (2). Thus, developing an optimal cerebral protection technique has been a cornerstone in the evolution of aortic arch surgery to obtain respectable outcomes.
History of retrograde cerebral perfusion (RCP)
Profound hypothermic circulatory arrest was introduced to aortic arch surgery in 1970s by Dr. Griepp and colleagues (3). Hypothermia minimizes the ischemic insults to the brain by suppressing its metabolic demand of glucose and oxygen. Circulatory arrest provided simplicity to the aortic arch surgery. However, safe duration of this technique soon became a concern. temporary neurologic dysfunction (TND) was often observed in patients with more than 25 minutes of profound hypothermic circulatory arrest, and its incidence was correlated with the duration of circulatory arrest by an odds ratio of 1.06 per minute (4). Svensson and colleagues reviewed outcomes of 656 patients and reported that the occurrence of PND significantly increased after 40 minutes of circulatory arrest time and the mortality rate significantly increased after 65 minutes of circulatory arrest (5). Thus, safe duration of profound hypothermic circulatory arrest time was recommended to limit up to 30 minutes.
To augment the cerebral protection during the profound hypothermic circulatory arrest, in 1990 Ueda and colleagues introduced continuous RCP technique, in which cold oxygenated blood was perfused to the brain via the superior cava (6). The original use of RCP was reported by Mills and Ochsner in 1980 to treat air embolisms during cardiopulmonary bypass, to flush out the air from the arch vessels (7). Ueda and colleagues modified the technique to a continuous usage and maintained a high internal jugular vein pressure of 20 mmHg to augment cerebral protection. The adjunctive use of continuous RCP theoretically allows to maintain cerebral hypothermia, prevent debris and air from reaching the terminal vessels of the brain, prevent microaggregation of blood cells, and delay the onset of acidosis in the ischemic brain by washing out the metabolites (6). In the late 1990s, multiple groups reported clinical studies demonstrating the safety and efficacy of the RCP with decreased stroke and mortality rate compared to the conventional profound hypothermic circulatory arrest (8,9). Our group showed that the use of RCP significantly reduced stroke rate from 9% to 4% in a series with median time circulatory arrest time of 42 minutes. Similarly, Coselli and colleagues demonstrated that stroke rate improved from 7% to 2% as well as 30-day mortality rate from 15% to 3% with RCP use (circulatory arrest time was not reported in this study) (9).
Recommended RCP pressure and flow
A canine animal study by Usui and colleagues (10) demonstrated that amount of blood return into the aortic arch had a linear relationship between jugular vein pressure between 15 and 25 mmHg, but jugular pressure beyond 25 mmHg was associated with an elevated cerebrospinal fluid pressure with no augmentation in return of the blood in the aortic arch. Similarly, Nojima and colleagues compared perfusion pressures of 10, 20, and 30 mmHg and concluded that retrograde flow from the arch vessels increased with venous pressure but significant cerebral edema and hyperemia occurred at 30 mmHg (11). Thus, maintenance pressure below 25 mmHg during RCP is a recommended perfusion pressure to avoid cerebral edema in the current practice.
Although excessive maintenance RCP flow causes cerebral edema, a transient higher pressure is required when initiating the RCP. Our group used transcranial power M-mode Doppler ultrasound during RCP to confirm the presence of reversed blood flow in the middle cerebral arteries, and we learned that “opening” pressure of 25 to 32 mmHg with RCP flow up to 1,500 mL/min for a very short period time was required to detect the reversal flow. After the blood flow in the middle cerebral arteries were observed, the RCP flow was then decreased to the maintenance flow rate below 500 mL/min with the superior vena cava line pressure below 25 mmHg and maintain the cerebral perfusion (12). We also found that snaring of the superior vena cava to isolate and clamping of the inferior vena cava cannula played an important role to control of superior vena cava pressure during RCP.
Cerebral monitoring during RCP
In addition to monitoring the superior vena cava pressure, the use of neurophysiological monitoring devices is crucial to obtain a sufficient but not excess RCP flow. We have demonstrated in the past that the use of transcranial power M-mode Doppler ultrasound monitoring guided RCP improved neurological outcomes by individualized settings of pump flow (12). However, just like any ultrasound evaluations, transcranial Doppler monitoring is highly operator dependent; a stable fixation of the Doppler probe for continuous monitoring is cumbersome; and some patients do not have appropriate bone windows for transcranial monitoring. Currently, we routinely use regional cerebral oxygenation by near-infrared spectroscopy (NIRS), placed at bilateral forehead to monitor the adequacy of RCP. Setting up NIRS is simple and the interpretation of the data does not require special training. In addition, findings of NIRS correlates well with information obtained with transcranial Doppler ultrasound (12). Thus, it is a preferred neurophysiological measuring method during aortic arch repair along with electroencephalogram today.
Safe duration of profound hypothermic circulatory arrest with RCP
Okita and colleagues in 1998 reported a single center experience with 148 aortic arch repairs and compared patients with circulatory arrest time under 60 minutes (CA <60, N=112) and over 60 minutes (CA ≥60, N=36). They found that the duration of RCP was not associated with early mortality or neurologic dysfunctions (hospital death, CA ≥60 13.8% vs. CA <60 8.9%, P=0.39; PND, CA ≥60 2.9% vs. CA <60 4.4%, P=0.65; TND, CA ≥60 25.0% vs. CA <60 25.0%, P=1.00) (13). Thus, they concluded that prolonged profound hypothermic circulatory arrest time, beyond 60 minutes, did not increase the risk of mortality and neurological dysfunctions when RCP was used to augment cerebral protection. The same group reported a prospective comparative control study of RCP (N=30) vs. antegrade cerebral perfusion (ACP, N=30) 3 years later (14), and reported similar PND rate (RCP 3.3% vs. ACP 6.6%, P=0.6) and mortality (RCP 6.6% vs. ACP 6.6%, P=1.0) between the 2 groups. However, 5 out of 6 patients who had cerebral circulatory arrest for more than 50 minutes in the RCP group developed severe TND. A small study (N=20) with mean RCP of 74 minutes reported by Sasaguri in 1996 documented the opposite results: only 1 of 6 patients whose RCP exceeded 90 minutes had neurological deficits (1 PND, 0 TND) (15). A recent study by Lau and colleagues (16) compared patients with circulatory arrest less than 50 minutes (CA <50, N=993) and over 50 minutes (CA ≥50, N=50). They found TND were significantly higher in patients with longer circulatory arrest time but mortality and PND rates were similar (mortality: CA <50 3.8% vs. CA ≥50 8.0%, P=0.143; PND, CA <50 1.2% vs. CA ≥50 2.0%, P=0.623; TND, CA <50 2.9% vs. CA ≥50 8.0%, P=0.045); In addition, when preoperative characteristics were matched between these 2 groups, there were no significant difference in the short-term outcomes [matched cohort mortality: CA <50 (N=48) 0.5% vs. CA ≥50 (N=48) 2.1%, P=0.257; matched cohort PND, CA <50 1.5% vs. CA ≥ 50 2.2%, P=0.749; TND, CA <50 2.0% vs. CA ≥50 6.4%, P=0.098]. Meanwhile, Usui and colleagues used multicenter database and demonstrated that the incidence of PND abruptly increased after RCP time exceeded 100 min (17). Thus, adjunctive RCP extend safe duration of profound hypothermic circulatory arrest to 50 minutes. RCP beyond 50 minutes may be acceptable in selected patients but should be applied with caution as there is a paucity of data to support its safety.
Comparison to ACP
In 1992, Kazui and colleagues reported a successful series of aortic arch replacement with selective ACP, inserting a balloon tipped perfusion catheter to the innominate and left common carotid arteries and clamping the left subclavian artery (18). Since then, there had been multiple debates on which cerebral protection, RCP or ACP, provides better outcomes. However, due to the complexity of aortic arch surgery, only 2 small prospective comparative studies for adjunctive cerebral protection measures have been published—and both are limited to total arch replacement: Okita and colleagues in 2001 allocated 60 patients undergoing total aortic arch replacement alternately to RCP and ACP, and reported that TND was significantly more prevalent in patients who received RCP than ACP, especially when circulatory arrest time was longer than 50 minutes, but there was no significant difference in the PND (6.6% in RCP, 3.3% in ACP) or mortality rate (6.6% in both group, P=1.00) (14); and Svensson, in 2015, reported a series of 121 patients and concluded that clinical stroke rate and death due to neurologic events was similar between 2 groups (PND, RCP 5.0% vs. ACP 0%, P=0.12; TND, RCP 1.7% vs. ACP 1.6%, P>0.9 death: 0% in RCP, 0% in ACP) (19).
Outcomes of major retrospective comparative studies for aortic arch repairs with RCP and ACP are detailed and summarized in Table 1. Studies by Sugiura and colleagues (20), Kaneko and colleagues (21), and Ganapathi and colleagues (22) focus on hemiarch replacement. All 3 studies concluded that incidence of TND/PND and mortality was similar between the 2 cerebral protection adjuncts (TND, RCP 0–38% vs. ACP 1–34%; PND, RCP 1–9% vs. ACP 3–12%; mortality, RCP 3–6% vs. 2–4%). Retrospective studies composed of combined hemiarch and total arch replacement or limited to total arch replacement demonstrated similar results, with no significant difference between RCP and ACP regarding rates of mortality, TND, or PND as shown in the Table 1 (19,23-28). Of note, circulatory arrest time in recent reports is relatively short compared to decades ago, and this may have biased the difference between the 2 cerebral protection adjuncts. In addition, one can argue that the number of patients in these studies is too small to conclude which cerebral protection is better. Recently, Okita et al. published one of the largest series on total arch replacement (27), comparing RCP and ACP after propensity match with 1,141 patients in each cohort. They found that there was no significant difference between these adjuncts with regard to incidence of 30-day mortality or PND/TND but shorter ICU stay in ACP group. Similarly, Englum and colleagues utilized Society of Thoracic Surgeons database and reviewed aortic arch repair with hypothermic circulatory arrest (36% elective, 8% total arch, median circulatory arrest time of 27 minutes), in which they found 4,418 cases with ACP and 3,149 with RCP for adjunctive cerebral protection strategies, and concluded that outcomes—including operative mortality and neurological complications—were similar between RCP and ACP (29). Thus, studies with patients greater than 1,000 in each arm of RCP and ACP failed to demonstrate the significant difference between the groups after aortic arch repair.
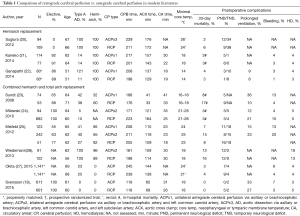
Full table
In conclusion, RCP and ACP provide equivalent results as adjunctive cerebral protection techniques to profound circulatory arrest during both the hemiarch replacement and total arch replacement when circulatory arrest time is limited to relatively short period.
Advantages of RCP
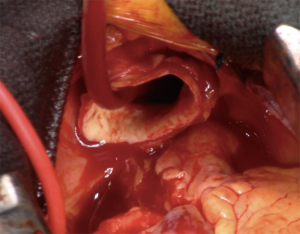
There are multiple advantages of RCP over ACP during hemiarch replacement. First, RCP does not require an exposure and manipulation of the aortic arch vessels, whereas ACP with right axillary/subclavian artery or direct balloon catheter perfusion to the arch vessels requires clamping or insertion of catheter to the arch vessels. Two-thirds of stroke after arch repair are thought to be of embolic origin while hypoperfusion accounts for one-third (30). Manipulations of the arch vessels potentially cause injury of the vessels or embolization of atherosclerotic debris. RCP not only reduces the risks of embolic stroke and but also potentially flushes the atheromatous debris out from the arch vessels. Second, surgery time and circulatory arrest time of RCP is shorter than ACP, as it does not require exposure of the axillary/subclavian artery or insertion of balloon-tipped catheters. Third, RCP does not interfere with surgical field (Figure 1) and distal anastomosis is easier compared to ACP.
Conclusions
There are no data that proves superiority of RCP or ACP during hemiarch repair. However, the safety of RCP has been proven in multiple studies up to 50 minutes of circulatory arrest time, which should be a long enough for a simple hemiarch replacement. When RCP is appropriately applied, a transient high opening pressure followed by maintenance perfusion pressure below 25 mmHg, it has proven to be as good as or better than ACP for hemiarch repair to augment cerebral protection during hemiarch replacement. This is due to its simplicity and better exposure of surgical field—with potentially lower risk of embolic events. Thus, we prefer RCP as the adjunct to profound hypothermic circulatory arrest for a hemiarch replacement.
Acknowledgements
None.
Footnote
Conflicts of Interest: ALE is a consultant for WL Gore. AT has no conflicts of interest to declare.
References
- Cooley DA, Mahaffey DE, De Bakey ME. Total excision of the aortic arch for aneurysm. Surg Gynecol Obstet 1955;101:667-72. [PubMed]
- Ergin MA, Griepp EB, Lansman SL, et al. Hypothermic circulatory arrest and other methods of cerebral protection during operations on the thoracic aorta. J Card Surg 1994;9:525-37. [Crossref] [PubMed]
- Griepp RB, Stinson EB, Hollingsworth JF, et al. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051-63. [PubMed]
- Ergin MA, Galla JD. Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J Thorac Cardiovasc Surg 1994;107:788-97. [PubMed]
- Svensson LG, Crawford ES, Hess KR, et al. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993;106:19-28; discussion -31.
- Ueda Y, Miki S, Kusuhara K, et al. Surgical treatment of aneurysm or dissection involving the ascending aorta and aortic arch, utilizing circulatory arrest and retrograde cerebral perfusion. J Cardiovasc Surg (Torino) 1990;31:553-8. [PubMed]
- Mills NL, Ochsner JL. Massive air embolism during cardiopulmonary bypass. Causes, prevention, and management. J Thorac Cardiovasc Surg 1980;80:708-17. [PubMed]
- Safi HJ, Letsou GV, Iliopoulos DC, et al. Impact of retrograde cerebral perfusion on ascending aortic and arch aneurysm repair. Ann Thorac Surg 1997;63:1601-7. [Crossref] [PubMed]
- Coselli JS, LeMaire SA. Experience with retrograde cerebral perfusion during proximal aortic surgery in 290 patients. J Card Surg 1997;12:322-5. [PubMed]
- Usui A, Oohara K, Liu TL, et al. Determination of optimum retrograde cerebral perfusion conditions. J Thorac Cardiovasc Surg 1994;107:300-8. [PubMed]
- Nojima T, Magara T, Nakajima Y, et al. Optimal perfusion pressure for experimental retrograde cerebral perfusion. J Card Surg 1994;9:548-59. [Crossref] [PubMed]
- Estrera AL, Miller CC 3rd, Lee TY, et al. Ascending and transverse aortic arch repair: the impact of retrograde cerebral perfusion. Circulation 2008;118:S160-6. [Crossref] [PubMed]
- Okita Y, Takamoto S, Ando M, et al. Mortality and cerebral outcome in patients who underwent aortic arch operations using deep hypothermic circulatory arrest with retrograde cerebral perfusion: no relation of early death, stroke, and delirium to the duration of circulatory arrest. J Thorac Cardiovasc Surg 1998;115:129-38. [Crossref] [PubMed]
- Okita Y, Minatoya K, Tagusari O, et al. Prospective comparative study of brain protection in total aortic arch replacement: deep hypothermic circulatory arrest with retrograde cerebral perfusion or selective antegrade cerebral perfusion. Ann Thorac Surg 2001;72:72-9. [Crossref] [PubMed]
- Sasaguri S, Yamamoto S, Hosoda Y. Time limitation of postural retrograde cerebral perfusion during cerebral hypothermic circulatory arrest. A report of an unsuccessful case. J Cardiovasc Surg (Torino) 1996;37:125-7. [PubMed]
- Lau C, Gaudino M, Iannacone EM, et al. Retrograde Cerebral Perfusion Is Effective for Prolonged Circulatory Arrest in Arch Aneurysm Repair. Ann Thorac Surg 2018;105:491-7. [Crossref] [PubMed]
- Usui A, Abe T, Murase M. Early clinical results of retrograde cerebral perfusion for aortic arch operations in Japan. Ann Thorac Surg 1996;62:94-103; discussion -4.
- Kazui T, Inoue N, Yamada O, et al. Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment. Ann Thorac Surg 1992;53:109-14. [Crossref] [PubMed]
- Svensson LG, Blackstone EH, Apperson-Hansen C, et al. Implications from neurologic assessment of brain protection for total arch replacement from a randomized trial. J Thorac Cardiovasc Surg 2015;150:1140-7 e11.
- Sugiura T, Imoto K, Uchida K, et al. Comparative study of brain protection in ascending aorta replacement for acute type A aortic dissection: retrograde cerebral perfusion versus selective antegrade cerebral perfusion. Gen Thorac Cardiovasc Surg 2012;60:645-8. [Crossref] [PubMed]
- Kaneko T, Aranki SF, Neely RC, et al. Is there a need for adjunct cerebral protection in conjunction with deep hypothermic circulatory arrest during noncomplex hemiarch surgery? J Thorac Cardiovasc Surg 2014;148:2911-7. [Crossref] [PubMed]
- Ganapathi AM, Hanna JM, Schechter MA, et al. Antegrade versus retrograde cerebral perfusion for hemiarch replacement with deep hypothermic circulatory arrest: does it matter? A propensity-matched analysis. J Thorac Cardiovasc Surg 2014;148:2896-902. [Crossref] [PubMed]
- Sundt TM 3rd, Orszulak TA, Cook DJ, et al. Improving results of open arch replacement. Ann Thorac Surg 2008;86:787-96; discussion -96.
- Milewski RK, Pacini D, Moser GW, et al. Retrograde and antegrade cerebral perfusion: results in short elective arch reconstructive times. Ann Thorac Surg 2010;89:1448-57. [Crossref] [PubMed]
- Misfeld M, Leontyev S, Borger MA, et al. What is the best strategy for brain protection in patients undergoing aortic arch surgery? A single center experience of 636 patients. Ann Thorac Surg 2012;93:1502-8. [Crossref] [PubMed]
- Wiedemann D, Kocher A, Dorfmeister M, et al. Effect of cerebral protection strategy on outcome of patients with Stanford type A aortic dissection. J Thorac Cardiovasc Surg 2013;146:647-55. e1.
- Okita Y, Miyata H, Motomura N, et al. A study of brain protection during total arch replacement comparing antegrade cerebral perfusion versus hypothermic circulatory arrest, with or without retrograde cerebral perfusion: analysis based on the Japan Adult Cardiovascular Surgery Database. J Thorac Cardiovasc Surg 2015;149:S65-73. [Crossref] [PubMed]
- Stamou SC, Rausch LA, Kouchoukos NT, et al. Comparison between antegrade and retrograde cerebral perfusion or profound hypothermia as brain protection strategies during repair of type A aortic dissection. Ann Cardiothorac Surg 2016;5:328-35. [Crossref] [PubMed]
- Englum BR, He X, Gulack BC, et al. Hypothermia and cerebral protection strategies in aortic arch surgery: a comparative effectiveness analysis from the STS Adult Cardiac Surgery Database. Eur J Cardiothorac Surg 2017;52:492-8. [Crossref] [PubMed]
- Gega A, Rizzo JA, Johnson MH, et al. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg 2007;84:759-66. [Crossref] [PubMed]
Cite this article as: Tanaka A, Estrera AL. Simple retrograde cerebral perfusion is as good as complex antegrade cerebral perfusion for hemiarch replacement. J Vis Surg 2018;4:50.