Video-assisted thoracoscopic PlasmaJet ablation for malignant pleural mesothelioma
Introduction
The role of surgery in malignant pleural mesothelioma (MPM) remains debatable, however the relative advantages of different surgical approaches are continuously reassessed and reconsidered (1). In carefully selected patients, extended pleurectomy and decortication procedures can provide macroscopic clearance of the disease and, when combined with chemotherapy, improve prognosis (2). For the remaining patients, surgery is performed mainly for palliation purposes, aiming to control symptoms and improve quality of life (3).
Minimally invasive debulking procedures, which could potentially be offered to more patients, have been proposed in the form of video-assisted thoracoscopic partial pleurectomy (VAT-PP) (4) or pleurectomy/decortication (VAT-PD) (5). Two studies have been designed in the UK to evaluate their outcomes: the MesoVATS, an open-label, randomised, controlled trial examined the role of VAT-PP versus talc pleurodesis in patients with MPM over a period of 10 years. Overall, the trial failed to demonstrate an effect on overall survival for VAT-PP compared to talc pleurodesis. Patients in the VAT-PP group experienced more complications and longer hospital stays, however there was some evidence of improvement in their quality of life parameters (6). The MesoTRAP, a pilot clinical trial and feasibility study currently in progress, aims to compare VAT-PD to indwelling pleural catheters (IPC), in order to determine the best method for palliation and symptomatic relief in patients with MPM presenting with pleural collections further complicated by a trapped lung (7).
Case presentation
We report on a case in which the PlasmaJet Surgery System (Plasma Surgical, UK) was used in our centre for purposes of cytoreduction and long-term symptomatic relief, in a patient diagnosed with MPM.
The PlasmaJet Surgery System is a device consisting of a console and a single-use hand piece, which releases a fine jet of argon plasma by heating pressurised argon gas. The use of kinetic energy and highly controlled thermal effects allows for better creation of dissection planes near sensitive structures (8), enables far-reaching removal of disease layer by layer (9), and facilitates sealing of areas with oozing surfaces, thus reducing the risk of leakages (10). Our aim was to examine a possible role for the PlasmaJet System in video-assisted surgery for mesothelioma, taking into consideration the benefits, as well as limitations of respective VATS approaches, as they have been identified in previous studies and clinical trials (11).
Patient selection and workup
A 76-year-old retired managing director, with a 30 pack-year smoking history and no known exposure to asbestos, presented to his local hospital in August 2017 with a history of cough and progressive breathlessness over the course of the previous three months. Weight loss was also reported, which the patient however attributed to diet. At his baseline, he was able to play three rounds of golf per week. His medical history was remarkable for hypertension, hypercholesterolaemia, gout and benign prostate hyperplasia.
He underwent removal of 1.4 L of straw coloured fluid, exudative as per Light’s criteria, which was later confirmed by cytology to be consistent with mesothelioma. Right pleural thickening and nodularity was evidenced on computed tomography. He was reviewed as an outpatient a week post-procedure, when early re-accumulation of fluid was diagnosed on a chest film, with accompanying clinical symptoms of breathlessness and severe pain in the right costodiaphragmatic recess, becoming worse with inspiration.
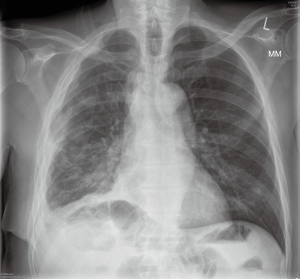
The patient was referred to our centre for surgical opinion and further management. He presented in our clinic two weeks following discharge from his local hospital, with symptoms of right sided chest pain and breathlessness, and a large right sided pleural effusion on his chest X-ray (Figure 1A). Following discussion with the patient regarding options for treatment, we offered him a video-assisted approach for drainage of the effusion, attempt for radical decortication using PlasmaJet ablation, and talc pleurodesis. Risks and benefits of the approach were thoroughly discussed and a mortality risk of 2–3% was quoted, to which the patient agreed.
Pre-operative preparation
We felt it was more appropriate to admit the patient in hospital the following day, for drainage of the large right-sided pleural effusion prior to his procedure, in order to relieve his breathlessness and re-assess his fitness for surgery. A small-bore drain was inserted using Seldinger’s technique and approximately 3 L of straw coloured fluid were gradually removed from the right hemithorax with rapid improvement of symptoms (Figure 1B). He was taken to theatres electively for his procedure.
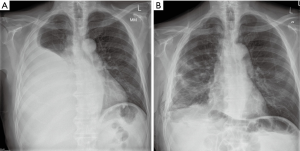
Procedure (Figure 2)

An access incision was performed at the level of the 5th intercostal space anterolaterally, and two ports were inserted through the 7th and 8th intercostal spaces, anteriorly and posteriorly. No fluid was found in the pleural cavity and a thickened pleura with multiple nodules on its surface, as well as nodularity on the surface of the diaphragm, pericardium and the lung were identified.
A patch of parietal pleura was mobilised with diathermy and removed. There was obvious invasion of the tumour in the deep layers of the chest wall with large nodules on the pleura; radical removal of the tumour was not attempted as it was deemed impossible.
Using PlasmaJet and blunt dissection, partial pleurectomy was performed. The rest of the pleura was ablated with the use of the PlasmaJet device to the level of visibly normal tissues. Multiple nodules on the diaphragmatic surface were also ablated down to muscle level, again with PlasmaJet.
The pleural cavity was washed with iodine solution and 7 g of sterile talc were insufflated in the pleural cavity with good cover. Apical and basal drains were inserted, the lung was re-inflated and the wound was closed in layers.
The patient was extubated in theatres and transferred to recovery for monitoring. He was then moved to the high dependency unit for overnight stay and the following morning was taken to the ward, where he remained until discharge. He was regularly reviewed by our physiotherapy team and pain nurse specialists.
Post-operative management
Both drains were connected to Rocket bottles (Rocket Medical, UK) and put on negative suction of 3 kPa following surgery, as per our routine management for pleurodesis procedures (Figure 3A). No air leak was identified in the immediate post-operative period and throughout the entire length of stay.
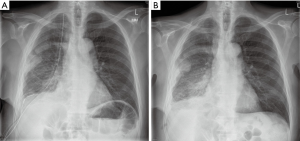
Fluid drainage on the first post-operative day was recorded at 250 mLs and pain was under good control. The patient was able to mobilise independently. To further encourage mobilisation, drains were connected to a Thopaz+ (Medela, Switzerland) portable drainage system with negative suction of 3 kPa.
Fluid drainage on second post-operative day was recorded at 55 mLs; both drains were kept in situ and on suction. Suction was removed on post-operative day three and, following confirmation of good lung expansion on a chest film, the apical drain was removed. Drainage on day three was recorded as 90 mls. On the fourth post-operative day only minimal drainage was recorded, the basal drain was removed (Figure 3B), and the patient was discharged home with a routine follow-up appointment in three weeks. Final histology confirmed biphasic mesothelioma.
The patient was advised to seek medical advice in case of deterioration while at home. He presented to his local hospital four days post-discharge, with uncontrolled pain on his right chest. His chest film confirmed stable post-operative appearances with good lung expansion and no fluid re-accumulation. He was discharged home with additional pain relief, which he still required by the time he was reviewed in our outpatient’s department. At that time, his chest X-ray, again, confirmed stable post-operative appearances, with a fully re-expanded right lung and no collections (Figure 4). He was referred to oncology for further management.
Tips, tricks and pitfalls
We advise against the use of the Yankauer plastic suction tip during this procedure because the material can be damaged when in contact with the jet of argon plasma; this generates dense smoke in the thoracic cavity which can obstruct the operative field. The use of a metal suction tip is preferable.
Discussion
As technical equipment advances, enabling surgical procedures to become less invasive, there is a constant need to re-evaluate our approach on managing diseases which bare a significant impact on patients’ lives. The PlasmaJet Surgery system is a new tissue sparing technology which allows for precise tissue dissection and ablation, while controlling intra-operative haemostasis, which may be able to achieve a comparable or even better outcome to standard pleurodesis procedures, without significantly affecting the rate of post-operative complications, overall hospital stay, time and costs.
We consider the procedure performed in this case study to have been successful, as it resulted to a satisfactory post-operative outcome with no associated post-operative complications. The patient’s overall stay in hospital was comparable to our talc pleurodesis procedures and it could even be reduced further in the future, as soon as we feel more confident in removing chest drains earlier. The procedure took more than two hours to complete and it involved the use of special equipment, which makes it more expensive than talc pleurodesis; this is something that will need to be examined in a more thorough manner, with regards to the long-term outcomes of the procedure.
To conclude, after demonstrating safety and absence of major adverse events with this approach, we feel justified in offering the procedure to more of our patients. We aim to prospectively collect data from future cases, which will allow us to retrospectively compare outcomes with those of talc pleurodesis alone. We, of course, understand that randomised surgical trials remain the only robust method to ascertain any new surgical procedure and we would strongly advocate the design of such a trial, if deemed appropriate by our preliminary results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this article and any accompanying images.
References
- Perikleous P, Waller DA. Video assisted thoracoscopic and open chest surgery in diagnosis and treatment of malignant pleural diseases. J Vis Surg 2017;3:85. [Crossref] [PubMed]
- Nakas A, Trousse DS, Martin-Ucar AE, et al. Open lung-sparing surgery for malignant pleural mesothelioma: the benefits of a radical approach within multimodality therapy. Eur J Cardiothorac Surg 2008;34:886-91. [Crossref] [PubMed]
- Martin-Ucar AE, Edwards JG, Rengajaran A, et al. Palliative surgical debulking in malignant mesothelioma. Predictors of survival and symptom control. Eur J Cardiothorac Surg 2001;20:1117-21. [Crossref] [PubMed]
- Waller DA, Morritt GN, Forty J. Video-assisted thoracoscopic pleurectomy in the management of malignant pleural effusion. Chest 1995;107:1454-6. [Crossref] [PubMed]
- Halstead JC, Lim E, Venkateswaran RM, et al. Improved survival with VATS pleurectomy-decortication in advanced malignant mesothelioma. Eur J Surg Oncol 2005;31:314-20. [Crossref] [PubMed]
- Rintoul RC, Ritchie AJ, Edwards JG, et al. Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014;384:1118-27. [Crossref] [PubMed]
- Rintoul RC. MesoTRAP pilot clinical trial and feasibility study. (2016). Available online: https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/mesotrap-pilot-clinical-trial-and-feasibility-study/
- Madhuri T, Butler S, Tailor A. Diaphragmatic surgery during debulking for epithelial ovarian cancer using argon plasma. ESGO Meeting 2013. (Poster Presentation). Available online: http://www.plasmasurgical.com/assets/6.html
- Sonoda Y, Olvera N, Chi DS, et al. Pathologic analysis of ex vivo plasma energy tumor destruction in patients with ovarian or peritoneal cancer. Int J Gynecol Cancer 2010;20:1326-30. [PubMed]
- Gugenheim J, Bredt LC, Iannelli A. A randomized controlled trial comparing fibrin glue and PlasmaJet on the raw surface of the liver after hepatic resection. Hepatogastroenterology 2011;58:922-5. [PubMed]
- Waller DA, Dawson AG. Randomized controlled trials in malignant pleural mesothelioma surgery—mistakes made and lessons learned. Ann Transl Med 2017;5:240. [Crossref] [PubMed]
- Perikleous P, Asadi N, Anikin V. Video-assisted thoracoscopic PlasmaJet ablation. Asvide 2018;5:131. Available online: http://www.asvide.com/article/view/23415
Cite this article as: Perikleous P, Asadi N, Anikin V. Video-assisted thoracoscopic PlasmaJet ablation for malignant pleural mesothelioma. J Vis Surg 2018;4:56.