Malperfusion syndromes in type A aortic dissection: what we have learned from IRAD
IntroductionOther Section
Type A acute aortic dissection (TAAD) is a disease that has a catastrophic impact on a patient’s life and emergent surgery represents the key goal of early treatment. Despite continuous improvements in diagnostic techniques and refinements in management strategies, surgical mortality still remains high, and is mostly influenced by patient clinical status at presentation (1). In TAAD patients, end-organ malperfusion is one of the most catastrophic complications that adversely influences outcomes (2-5). It has an incidence ranging from 16% to 34%, and may involve any of the major arterial side branches resulting in myocardial, cerebral, spinal cord, visceral and/or limb ischemia (2,6,7). In patients with malperfusion, the optimal therapeutic management is controversial, and several issues remain under debate. In this setting, the International Registry of Acute Aortic Dissection (IRAD), one of the largest worldwide registries for acute aortic dissection enrolling patients at major aortic referral centers, represents an ideal tool for evaluating clinical characteristics, management, and outcomes of TAAD patients. The present review aimed to assess current evidence on TAAD patients with the complication of malperfusion, as enunciated by the IRAD investigators.
Evidence from IRADOther Section
In the original IRAD study cohort, signs and symptoms of malperfusion were reported in 20–30% of patients and were associated with poorer outcomes (8,9). Patients who died during hospitalization suffered from an increased rate of malperfusion complications, such as neurological deficits (24% vs. 15%), myocardial ischemia (15% vs. 9%), visceral ischemia (6% vs. 2%), renal failure (11% vs. 3%), and limb ischemia (14% vs. 7%), compared with survivors (P<0.05) (9).
The presence of pulse deficits has long been recognized as a marker for malperfusion (10). This sign implies that major aortic side branches are compromised by the intimal flap and/or by compression of the true lumen, and thus may identify a subgroup of patients more likely associated with brain, visceral, or limb malperfusion. Pulse deficits were detected in nearby a third of IRAD patients and was found to be an independent predictor of early mortality (10). Hospital mortality varied substantially according to the number of vessels involved; 24.7% in patients with no pulse deficits, and 36.2%, 48.9% and 55.9% in patients who had decreased or absent pulsation in 1, 2 and 3 vessels, respectively (P<0.001). Similarly, in-hospital adverse events occurred more frequently in the group with pulse deficits. Neurologic deficits (35% vs. 11%) and coma (27% vs. 9.1%) were threefold greater, renal failure 2 times higher (10% vs. 4.6%) and limb ischemia almost 14 times more frequent (29% vs. 2.1%) in patients presenting with pulse deficits than in those without. Furthermore, patients with pulse deficits were more likely to have hypotension at presentation (10,11). The latter, defined as a systolic blood pressure ≤90 mmHg, was documented in >25% of IRAD patients and was associated with a much higher rate of malperfusion complications and in-hospital mortality (55% vs. 10%, P<0.001). In a series of 1,073 patients with acute aortic dissection, Tsai et al. (11) reported an incidence of neurologic deficits, myocardial ischemia, mesenteric ischemia and limb ischemia of 23%, 15%, 7% and 15%, respectively, in patients with hypotension compared with 12%, 7%, 3%, 7% and 10%, respectively, in patients presenting with no hypotension (P<0.001) (11). Thus, IRAD data suggest that the occurrence of both pulse deficits and hypotension correlate with malperfusion complications and should move caregivers toward timely surgical or percutaneous interventions to re-establish blood flow to vital organs.
In TAADs complicated by end-organ ischemia, a timely diagnosis and characterization of the type and the extent of malperfusion is crucial in determining optimal therapy and likely patient outcomes. Prolonged time intervals between the initial symptoms of TAAD and confirmation of the diagnosis with subsequent treatment will affect in a greater likelihood of irreversible end-organ ischemia with poor patient prognosis. As expected, in IRAD, early mortality in such unstable patients was much higher compared to patients without unstable features (31.4% vs. 16.7%), regardless of the type of intervention. Time to operation was a predictive factor of survival in this high-risk category of patients. Not surprisingly, mean time interval from onset of symptoms to surgical intervention was shorter in unstable patients than in stable patients (3.4 vs. 5 hours) (1).
Myocardial malperfusion
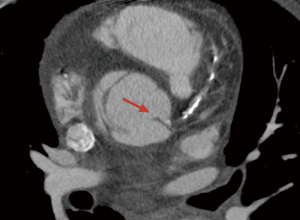
Coronary malperfusion complicates 10–15% of TAAD cases (2,12-14). It may be the result of hypotension, extension of the aortic dissection into a coronary artery ostium, a dynamic flap occlusion at the level of the coronary sinuses covering the coronary ostia in diastole, pre-existing coronary disease, or a combination of these (Figure 1). In IRAD, ischemic electrocardiogram (ECG) abnormalities were observed in 17.3% of TAAD, and findings of myocardial infarction (new Q waves or ST segments) in 7.1% (8). Consistent with data reported by others (15,16), IRAD investigators showed that ECG changes consistent with an acute coronary syndrome leads to delays in diagnosis in TAAD. The median time from presentation to diagnosis was 4 hours in patients with a normal ECG and almost 24 hours in patients presenting with ischemic ECG abnormalities (17). Moreover, when comparing patients undergoing early TAAD repair (<24 hours from symptom onset) with those having late interventions (>24 hours), the latter group exhibited a higher incidence of coronary malperfusion such as myocardial infarction (11.2% vs. 3%) and ECG evidence of new Q waves and ST elevations (15.3% vs. 8%). In addition to the delay in surgical treatment, the finding of ECG ischemic abnormalities, may initially mislead clinicians into considering a possible acute aortic syndrome case as the most likely diagnosis which than may have potentially deleterious consequences by exposing the patient to inappropriate, and potentially harmful, treatments with antithrombotic agents (16). In this setting, clinical and laboratory findings have been proposed to facilitate the timely and accurate diagnosis of TAAD. An aortic dissection detection risk score for identification of acute aortic dissection at initial presentation was created on the basis of several clinical risk markers reported in the 2010 American Heart Association and American College of Cardiology guidelines (18). In the IRAD study cohort, this diagnostic screening tool demonstrated satisfactory sensitivity (>95%) to capture the vast majority of patients presenting with TAAD (19). Moreover, the IRAD Substudy on Biomarkers (IRAD-Bio study) evaluated the diagnostic performance of D-dimer in a population of patients with chest pain and a suspicion for aortic dissection. Initial results showed that D-dimer levels were markedly elevated in TAAD, and that it may be useful in risk stratifying patients with suspected aortic dissection to rule out TAAD (cutoff value: 0.5 µg/mL), if used within the first 24 hours after symptom onset (20).
In TAAD, the presence of cardiac malperfusion has been associated with poor surgical outcomes (1,3,4,21). In a series of 682 IRAD patients undergoing surgical repair, Rampoldi et al. (21) revealed that myocardial ischemia and infarction (OR 1.76) and the necessity to perform coronary revascularization (OR 2.54) were independent preoperative predictors of mortality. Not surprisingly, preoperative left and/or right ventricular dysfunction were also strongly associated with high surgical mortality (1). However, while myocardial malperfusion carries an increased risk of operative mortality, timely intervention restoring coronary perfusion is the only viable treatment in these critically ill patients, and surgical aortic repair still remains the treatment of choice. In this setting, emergency percutaneous coronary intervention (PCI) may be considered as a treatment option, for very selected patients, as a bridge to surgery (13). PCI can be technically challenging and potentially time consuming in TAAD, and has no effect in treating the on-going dissection process, may involve further injury of the aortic wall and requires post-procedure antithrombotic medications that result in increased risk of bleeding, rupture and/or tamponade (16).
Cerebral malperfusion
Cerebral malperfusion occurs in 6–14% of TAAD patients and results from partial or complete occlusion of the arch vessels by the intimal-medial flap, hypoxic encephalopathy secondary to shock or tamponade and/or brain embolism from thrombus in the false lumen (Figure 2) (2-4,14,22). Clinical manifestations of stroke or coma were shown to be predictors for detrimental outcomes, and optimal management of TAAD patients with cerebral malperfusion syndrome remains controversial. IRAD data showed that nearly 1 out of 10 TAAD patients are complicated by major brain injury at the onset of dissection [cerebrovascular accident (CVA) 4.7%; coma 2.9%] (22). Patients presenting with coma were more hemodynamically compromised with a greater incidence of hypotension, shock and/or tamponade leading to end-organ dysfunction. The imaging findings were similar in patients with and without brain injury, except for arch vessel involvement by the dissection which was more likely in CVA patients (62%) compared to coma patients (44%) and uncomplicated patients (36%) (P<001). Such differences strongly influenced patient survival carrying a two or threefold higher mortality for patients with CVA and coma, respectively (CVA 40%; coma 60%; no brain injury 23%, P<0.001).
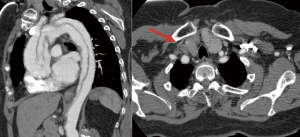
Major brain injury at presentation has long been considered a contraindication to emergent surgery with several authors proposing a delayed surgical approach after the neurologic status has improved (4,23,24). At IRAD enrolling centers, presence and type of brain injury clearly influenced patient management. Surgery was not performed in 24% of patients with CVA and 33% of patients with coma, compared with 11% of patients without brain injury. However, when assessing hospital outcomes according to therapeutic management, the investigators showed that medical therapy was associated with dismal outcomes: 100% mortality in patients with coma and 76.2% in those with CVA. Conversely, surgery was found to be a protective factor against mortality (OR 0.058; P<0.001), leading to a 50% survival benefit over medical management.
Since the brain is the organ most prone to ischemic damage, in TAAD with major brain injury, probably more than in other malperfusion syndromes, minimizing the ischemic time is crucial to increase the chances of a successful neurologic recovery. Over the last decade, a number of reports have documented the value of emergent aortic repair suggesting a cutoff value of 9–10 hours for predicting lack of neurologic improvement (24-26). Nevertheless, IRAD data revealed that, despite longer interval times from symptoms to surgery (CVA 12.3 hours, coma 13.8 hours), CVA and coma resolved in 84% and 79% of patients. Moreover, evidence of reversal of brain injury was a protective factor against mortality, in the surgically managed population (22).
Therefore, the observations coming from IRAD, indicate that TAAD patients with neurologic injury should always be considered for intervention, especially if early surgery is feasible and there are no signs of neurologic devastation.
Mesenteric malperfusion
Mesenteric malperfusion is among the most insidious and detrimental forms of ischemic end-organ complications occurring in TAAD (Figure 3). The incidence of mesenteric ischemia has been reported to be approximately 4–6%, in large multicenter registries (2,27). In TAADs complicated by mesenteric malperfusion, diagnostic and management decision-making is frequently challenging and remains controversial. IRAD data (27) showed that patients presenting with mesenteric malperfusion were more likely to have abdominal, leg and migrating pain compared with those who did not experience visceral malperfusion. However, abdominal pain did not occur in more than 40% of patients with mesenteric ischemia, whereas about 20% of patients without mesenteric malperfusion had pain. Thus, abdominal pain, while important, is a non-specific symptom of acute mesenteric ischemia (28). Mesenteric malperfusion was frequently associated with clinical or imaging signs of other organ injuries, such as coma (10%), ischemic spinal cord damage (6.8%), acute renal failure (52.2%) and limb ischemia (38.5%), that may further complicate and delay the diagnostic process (27). Indeed, at IRAD centers, an overall higher number of imaging modalities were required to diagnose TAAD in patients with mesenteric malperfusion. While, computed tomography scan, magnetic resonance imaging and transesophageal echocardiography were used with similar frequency to assess characteristics of dissection in patients with and without mesenteric malperfusion, angiography was more frequently performed in the malperfusion group (33.3% vs. 11.0%). Despite that, the time delay between symptom onset and both diagnosis and surgery were similar in patients with and without mesenteric ischemia (27).
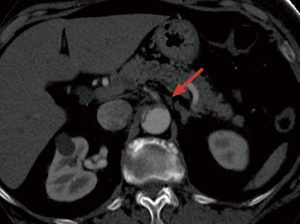
TAAD complicated by mesenteric malperfusion has been associated with extremely poor outcomes (29). In IRAD, almost two-thirds of patients died during hospitalization, almost a 3-fold increase compared to those patients without malperfusion (63.2% vs. 23.8%, P<0.001). Furthermore, mesenteric malperfusion has been shown to be one of the strongest risk factors for early mortality (OR 2.5) (27).
In the setting of visceral malperfusion, the correct management and timing of aortic repair is still a subject of debate. While there is general agreement that early reperfusion is critical for mesenteric malperfusion, it is not clear whether initial central aortic repair or percutaneous and/or extra-anatomic reperfusion best accomplishes that end (3,30,31). Some groups, given the unpredictable nature of TAAD and its potential for rupture, suggest immediate central aortic repair followed by investigation and treatment of residual malperfusion (3,32). Conversely, other authors, in selected patients with established visceral ischemic dysfunction, recommend initial catheter-based end-organ reperfusion followed by delayed central aortic repair (4,31). At IRAD centers, patients presenting with mesenteric malperfusion were less likely to undergo surgical/hybrid treatment (53% vs. 88%) and more likely to receive endovascular (16% vs. 1%) or medical (31% vs. 12%) management, compared to uncomplicated patients. These data undoubtedly reflect a resistance of surgeons to proceed with open surgery in such patients. At the same time, when assessing hospital mortality according to different therapeutic management, surgical/hybrid therapy was associated with superior clinical outcomes; in-hospital mortality was 41.7%, 72.7% and 95.2%, in patients who underwent surgical/hybrid, endovascular, and medical treatment, respectively (P<0.001). In addition, surgical/hybrid management emerged as a protective factor for early mortality in patients deemed operable by IRAD investigators. This is likely due to both patient selection and the potential benefit of definitive aortic repair. However, hybrid management (central aortic operation plus percutaneous treatment of mesenteric malperfusion) was performed in only a very few cases, and central aortic repair still represents the most common therapeutic approach, in this setting (27). Yet, when visceral ischemia is clinically manifest and advanced, percutaneous fenestration with or without stenting to reperfuse the ischemic organs, as an initial procedure, may be more likely to achieve patient survival and prevent ineffective open aortic repair, in extremely high-risk individuals. Contrariwise, in patients with malperfusion but no significant advanced end-organ dysfunction, proximal repair should occur first (31). Thus, the complexities of management in such patients must require a prompt referral to dedicated multidisciplinary teams involving cardiac, endovascular and vascular surgeons equipped with a full array of interventional, hybrid, and surgical techniques (33).
ConclusionsOther Section
Patients presenting with TAAD complicated by malperfusion syndromes represent one of the highest surgical risk cohorts for cardiovascular surgeons. In this subgroup of patients surgical outcomes remains poor, especially in those with mesenteric ischemia. Nevertheless, when compared with other forms of therapy, early central aortic repair appears to be associated with superior clinical outcomes and thus should be considered in all patients. However, the optimal management should be individualized for each patient based on presenting characteristics, type of malperfusion and time to surgery.
AcknowledgementsOther Section
IRAD is supported by grants from Gore Medical Inc. (Flagstaff, AZ, USA), Medtronic, Inc. (Minneapolis, MN, USA), Terumo Medical (Tokyo, Japan), The Hewlett Foundation, Ann and Robert Aikens, the University of Michigan Health System, the Varbedian Fund for Aortic Research, and the Mardigian Foundation.
FootnoteOther Section
Conflicts of Interest: The authors have no conflicts of interest to declare.
ReferencesOther Section
- Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg. 2005;129:112-22. [Crossref] [PubMed]
- Czerny M, Schoenhoff F, Etz C, et al. The Impact of Pre-Operative Malperfusion on Outcome in Acute Type A Aortic Dissection: Results From the GERAADA Registry. J Am Coll Cardiol. 2015;65:2628-35. [Crossref] [PubMed]
- Geirsson A, Szeto WY, Pochettino A, et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg 2007;32:255-62. [Crossref] [PubMed]
- Girdauskas E, Kuntze T, Borger MA, et al. Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2009;138:1363-9. [Crossref] [PubMed]
- Narayan P, Rogers CA, Benedetto U, et al. Malperfusion rather than merely timing of operative repair determines early and late outcome in type A aortic dissection. J Thorac Cardiovasc Surg. 2017;154:81-6. [Crossref] [PubMed]
- Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol. 2011;58:2455-74. [Crossref] [PubMed]
- Pacini D, Leone A, Belotti LM, et al. Acute type A aortic dissection: significance of multiorgan malperfusion. Eur J Cardiothorac Surg. 2013;43:820-6. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type A aortic dissection. Circulation. 2002;105:200-6. [Crossref] [PubMed]
- Bossone E, Rampoldi V, Nienaber CA, et al. Usefulness of pulse deficit to predict in-hospital complications and mortality in patients with acute type A aortic dissection. Am J Cardiol. 2002;89:851-5. [Crossref] [PubMed]
- Tsai TT, Bossone E, Isselbacher EM, et al. Clinical characteristics of hypotension in patients with acute aortic dissection. Am J Cardiol. 2005;95:48-52. [Crossref] [PubMed]
- Neri E, Toscano T, Papalia U, et al. Proximal aortic dissection with coronary malperfusion: presentation, management, and outcome. J Thorac Cardiovasc Surg. 2001;121:552-60. [Crossref] [PubMed]
- Imoto K, Uchida K, Karube N, et al. Risk analysis and improvement of strategies in patients who have acute type A aortic dissection with coronary artery dissection. Eur J Cardiothorac Surg. 2013;44:419-24; discussion 424-5. [Crossref] [PubMed]
- Immer FF, Grobéty V, Lauten A, et al. Does malperfusion syndrome affect early and mid-term outcome in patients suffering from acute type A aortic dissection? Interact Cardiovasc Thorac Surg. 2006;5:187-90. [Crossref] [PubMed]
- Rapezzi C, Longhi S, Graziosi M, et al. A. Risk factors for diagnostic delay in acute aortic dissection. Am J Cardiol. 2008;102:1399-406. [Crossref] [PubMed]
- Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol. 2007;99:852-6. [Crossref] [PubMed]
- Harris KM, Strauss CE, Eagle KA, et al. Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2011;124:1911-8. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266-369. [Crossref] [PubMed]
- Rogers AM, Hermann LK, Booher AM, et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation. 2011;123:2213-8. [Crossref] [PubMed]
- Suzuki T, Distante A, Zizza A, et al. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation. 2009;119:2702-7. [Crossref] [PubMed]
- Rampoldi V, Trimarchi S, Eagle KA, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83:55-61. [Crossref] [PubMed]
- Di Eusanio M, Patel HJ, Nienaber CA, et al. Patients with type A acute aortic dissection presenting with major brain injury: should we operate on them? J Thorac Cardiovasc Surg. 2013;145:S213-21.e1. [Crossref] [PubMed]
- Tanaka H, Okada K, Yamashita T, et al. Surgical results of acute aortic dissection complicated with cerebral malperfusion. Ann Thorac Surg. 2005;80:72-6. [Crossref] [PubMed]
- Estrera AL, Garami Z, Miller CC, et al. Acute type A aortic dissection complicated by stroke: can immediate repair be performed safely? J Thorac Cardiovasc Surg. 2006;132:1404-8. [Crossref] [PubMed]
- Morimoto N, Okada K, Okita Y. Lack of neurologic improvement after aortic repair for acute type A aortic dissection complicated by cerebral malperfusion: predictors and association with survival. J Thorac Cardiovasc Surg. 2011;142:1540-4. [Crossref] [PubMed]
- Tsukube T, Hayashi T, Kawahira T, et al. Neurological outcomes after immediate aortic repair for acute type A aortic dissection complicated by coma. Circulation. 2011;124:S163-7. [Crossref] [PubMed]
- Di Eusanio M, Trimarchi S, Patel HJ, et al. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: observations from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg. 2013;145:385-90.e1. [Crossref] [PubMed]
- Howard TJ, Plaskon LA, Wiebke EA, et al. Nonocclusive mesenteric ischemia remains a diagnostic dilemma. Am J Surg. 1996;171:405-8. [Crossref] [PubMed]
- Perera NK, Galvin SD, Seevanayagam S, et al. Optimal management of acute type A aortic dissection with mesenteric malperfusion. Interact Cardiovasc Thorac Surg. 2014;19:290-4. [Crossref] [PubMed]
- Yamashiro S, Arakaki R, Kise Y, et al. Management of visceral malperfusion complicated with acute type A aortic dissection. Interact Cardiovasc Thorac Surg. 2015;21:346-51. [Crossref] [PubMed]
- Deeb GM, Patel HJ, Williams DM. Treatment for malperfusion syndrome in acute type A and B aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg. 2010;140:S98-100. [Crossref] [PubMed]
- Estrera AL, Huynh TT, Porat EE, et al. Is acute type A aortic dissection a true surgical emergency? Semin Vasc Surg. 2002;15:75-82. [Crossref] [PubMed]
- Tsagakis K, Konorza T, Dohle DS, et al. Hybrid operating room concept for combined diagnostics, intervention and surgery in acute type A dissection. Eur J Cardiothorac Surg. 2013;43:397-404. [Crossref] [PubMed]
Cite this article as: Berretta P, Trimarchi S, Patel HJ, Gleason TG, Eagle KA, Di Eusanio M. Malperfusion syndromes in type A aortic dissection: what we have learned from IRAD. J Vis Surg 2018;4:65.