Coarctation repair—redo challenges in the adults: what to do?
Introduction
Coarctation of the aorta is defined as a congenital stenosis of the aorta, most commonly located at the juxtaductal position. It was first described by Morgagni in 1760 (1). Coarctation can occur as a solitary pathology limited to the aortic isthmus, or can present as a more complex lesion including long segment hypoplasia of the transverse aortic arch, or stenosis of the abdominal aorta (2-4). Other associated cardiac pathologies include bicuspid aortic valve, ventricular septal defect, patent ductus arteriosus, transposition of the great arteries, atrioventricular canal defects and hypoplastic left heart syndrome (5-9). Coarctation is a common congenital defect and accounts for approximately 5% to 7% of all congenital cardiac defects (10). The approximated incidence of coarctation is 3 cases per 1,000 births (11). Patients who do not undergo treatment suffer from hypertension, heart failure and aortic aneurysm, and have an increased risk for early mortality (12).
The first successful surgical repair of aortic coarctation was performed by Crafoord in Sweden and Gross in the United States in 1945 via a left lateral thoracotomy (13,14). The procedure involved resection of the stenosed aortic segment followed by direct end-to-end anastomosis of the transected aortic segments. However, direct anastomosis of the transected aortic segments leads to a high re-coarctation rate, especially when applied to neonates (15-18). This triggered the introduction of alternative surgical approaches, including the subclavian flap angioplasty, the usage of a prosthetic patch, and prosthetic interposition grafting. Nowadays, the extended end-to-end anastomosis is probably the most commonly applied technique with the lowest rate for re-coarctation over long-term follow up. Depending on the applied technique, the aortic re-coarctation rate is reported to be 10% to 41% (16,19,20). Another potential late complication after coarctation repair is the formation of an aortic aneurysm in the previously treated aortic segment. The incidence of aneurysm formation following coarctation repair has been reported to be between 5% and 51% (21-26).
In addition to conventional open repair, endovascular balloon angioplasty and stent graft placement have emerged as treatment alternatives for older children and adults with discrete area of coarctation. Late complications such as aneurysm formation or development of endoleaks can occur after these endovascular treatments, which may need further interventions. In addition to recurrent coarctation and aneurysm formation, persistent hypertension may also require redo surgery.
Given high late complication rates necessitating reintervention in 5% to 50% of cases, redo repair of previous aortic coarctation represents an important field of aortic surgery. Both endovascular and conventional open repair play important roles in the treatment of late complications after previous coarctation repair. This article will review the incidence of late complications after coarctation repair and will discuss the treatment options for redo coarctation repair in adult patients.
Initial operative techniques and associated incidence of recurrent coarctation
Initial surgical or endovascular repair of aortic coarctation yields excellent short-term results, however long-term complications include aneurysm formation and re-coarctation. The occurrence of both complications is dependent on the initial method for coarctation repair.
End-to-end anastomosis
Crafoord and Gross described the first successful surgical repair in 1945 via a left lateral thoracotomy (13,14). The aorta is adequately mobilized and cross clamped proximal and distal to the stenosed segment. A proximal aortic transection is made to resect the stenosed isthmus and the coarcted segment. A second aortic transection is made distal to the coarctation. The remaining aortic arch and the descending aorta are subsequently brought together with a direct end-to-end anastomosis. The incidence of re-coarctation is relatively high following this initial repair, with reported incidence rates of 41% to 51% (15-18). The occurrence of re-coarctation is age-dependent and is highest if the surgery is performed in neonates. Given the high incidence of re-coarctation, direct end-to-end anastomosis is not commonly used nowadays.
Prosthetic patch aortoplasty
Due to the high re-coarctation rate following the end-to-end anastomosis technique, physicians sought alternative surgical options to treat aortic coarctation. Vosschulte was the first surgeon who described the usage of a prosthetic patch to augment the aorta (27). After a longitudinal incision of the diseased segment, a prosthetic patch is sutured across the incision to augment this segment. Although the usage of Dacron grafts resulted in a lower rate of re-coarctation (28), a high incidence of aortic aneurysm formation was observed with this technique (20% to 40%). The usage of polytetrafluoroethylene reduced the rate of aneurysm formation to 7%, but led to a higher re-coarctation rate (25%) instead (29). For these reasons, patch aortoplasty has mostly been abandoned for the treatment of simple aortic coarctation. However, it is still being used for complex cases that require aortic arch reconstruction.
Subclavian flap aortoplasty
In this technique initially described by Waldhausen and colleagues in 1966 (30), the subclavian artery is ligated close to the origin of the left vertebral artery. The flap is generated by an incision of the subclavian artery that is extended down onto the aortic isthmus and across the stenosed segment. The flap is then folded downwards and sutured into the incised aorta, enlarging the previously stenosed aorta. The re-coarctation rate of this technique seems to be relatively low when performed in older children [0–3% (31,32)]. However, when applied to neonates, re-coarctation may occur in up to 23% (33). Although sacrificing the subclavian artery does not result in left arm ischemia, it may cause claudication in the long term (31).
Extended end-to-end anastomosis
Amato described this technique in 1977 and the procedure is frequently applied nowadays (34). In contrast to a direct end-to-end anastomosis, the proximal clamp is placed across the aortic arch including the left subclavian or even the left carotid artery including the aortic arch. Distally, the aorta is clamped below the stenosed segment. After ligation and division of the ductus arteriosus, the coarctation segment is resected and the aortic arch is opened on its inferior aspect, followed by end-to-end anastomosis of the opened arch and the descending aorta. The procedure can be performed with low peri-operative mortality and reports show relatively low re-coarctation rates of 4% to 13% (19,35-40).
Interposition graft
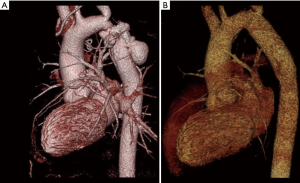
Described by Gross in 1951 (41), this technique is based on the resection of the coarctation segment, followed by a Dacron tube graft or aortic homograft interposition (Figure 1). Since non-native tissue is used, it does not grow with the patient and is therefore primarily reserved for patients in whom outgrowth of the graft is not an issue. The short- and long-term results in adults are respectable: Yousif and colleagues reported a peri-operative mortality of 0% and no re-coarctation events during mean follow-up of 10±7.6 years (42). Comparable outcome has been reported by other groups (43).
Extra-anatomical correction
This technique aims to provide additional blood flow to the distal aorta, however leaves the stenosed aorta in situ. This is generally performed via a median sternotomy, with cardiopulmonary bypass support (44,45). Proximally, a prosthetic conduit is anastomosed to the ascending aorta or the subclavian artery (46) and is anastomosed to the descending aorta distally, thereby bypassing the aortic coarctation segment. This technique is especially useful when concomitant cardiac procedures (such as aortic valve replacement or coronary artery bypass grafting) need to be performed as well (47).
Balloon angioplasty
Transcatheter balloon angioplasty was first described by Singer in 1982 (48). Access is obtained via the femoral artery and a balloon catheter is advanced up to the obstructed segment. There, the balloon is inflated to enlarge the aortic diameter. Short-term results are acceptable in infants; however, the long-term outcome is less favorable, with re-coarctation rates of up to 80% (49-52). In adolescents, the long-term complication rate of up to 50% have been reported for re-coarctation and aneurysm formation (53-55). Given this, balloon angioplasty might be considered an alternative for subtle coarctation in adolescents, but cannot be considered a curative approach in neonates. Therefore, in patients with native aortic coarctation, transcatheter balloon angioplasty should only be used to stabilize critically ill infants as a bridge to anticipated surgery.
Stent implantation
Both bare metal stents and covered endografts have been used to treat coarctation. Endovascular stent graft placement offers the benefit of a less invasive approach and a potentially quicker recovery. The procedure can be performed with a low perioperative risk. Stent implantation has replaced balloon angioplasty for discrete coarctation in adolescents and adults in most centers nowadays. Stent implantation is generally not advised in patients of less than 25 kg of body weight. In these patients, there is an increased risk for potential femoral artery rupture due to the sheath size. Furthermore, the native aorta is relatively small and may outgrow the stent, thus requiring reintervention for transcatheter dilation of the stent (56,57). The re-coarctation rates seem to be comparable with open surgical approaches (58-62). However, aneurysm formation has been observed in 6% of cases after stent graft placement (58-61). It has been previously suggested that bare metal stents might have a tendency towards future development of (pseudo) aneurysms (63-65). However, a more recent study comparing the two stent types did not observe any significant difference (66).
Factors predisposing to re-coarctation and aneurysmal degeneration
An overview of recurrence rates associated with initial operative techniques can be found in Table 1. Although the incidence of re-coarctation is primarily dependent on the initial treatment approach, there are other factors that predispose to recurrent coarctation. Patient age (especially infants <30 days of age) (69), coarctation segment diameter (69), and isthmus hypoplasia (69,70) have been associated with increased incidence of re-coarctation. A diameter of 3.5 mm or less prior to angioplasty, or a diameter of 6 mm or less post-intervention have been reported to be associated with an increased risk for recurrent coarctation (69).
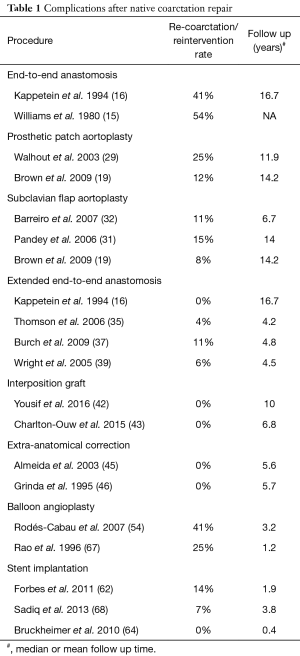
Full table
Aneurysmal degeneration can occur following both surgical or endovascular coarctation repair. The pathophysiological development of an aneurysm after coarctation repair is dependent on abnormal aortic tissue at the coarctation site. The pathological aorta’s medial layer involves fragmented elastic fibers, a higher amount of ground substance, and fewer smooth muscle cells (71) that can deteriorate over time. It has been suggested that an aneurysm may occur if all the pathological tissue is not resected during open surgical repair (72,73). Likewise, balloon angioplasty leaves the abnormal aortic tissue in place, thus promoting potential future aneurysm formation.
The incidence of aneurysm formation varies over a wide range. While some studies report a re-coarctation rate of 0% after surgical repair with the resection and end-to-end anastomosis technique, the rate can be as high as 20–40% following prosthetic patch aortoplasty (21,22,28,29,74). Furthermore, the patient age at the time of the first coarctation repair is a predisposing factor for aneurysm formation, with a higher incidence observed in children aged 13.5 years or older at the time of repair.
Indications for reintervention after coarctation repair
Recurrent coarctation or re-coarctation is defined as restenosis after previous successful surgical or endovascular repair of native aortic coarctation. Clinical symptoms that may indicate re-coarctation include hypertension and headache. The indications for intervention of recurrent coarctation resemble those for native coarctation (75-78), and include persistent hypertension, a peak pressure gradient of 20 mmHg or more, and evidence of collateral circulation on imaging (76-78). Besides re-coarctation, aneurysm formation is another complication after coarctation repair that may require reintervention. The aneurysm usually develops at the site of the previous repair, but occasionally involves the ascending aorta, especially in the setting of a bicuspid aortic valve (79,80). The societal guidelines for aortic coarctation do not clearly define indications for the treatment of aneurysm formation after coarctation, however, one may refer to the guidelines for the management and treatment of thoracic aortic aneurysms (81,82). For aortic arch aneurysms, intervention is recommended at an aortic diameter of 55 mm or more. For descending aortic aneurysms, intervention is recommended for aortic diameter of 55 mm or more if treatment with endovascular modalities is feasible. If endovascular treatment is not possible, open surgical repair is indicated in patients with an aortic diameter of 60 mm or more. Both surgical and endovascular repair are applicable therapeutic options for re-coarctation. Various factors should be considered when evaluating a patient with re-coarctation, including the patient’s age and general condition as well as the localization and extent of the aneurysm and feasibility for endovascular repair.
Techniques for re-intervention
The current guidelines of the European and American societies recommend an endovascular approach as the initial treatment strategy for recurrent coarctation (75-78). Conventional surgery is recommended for complicated cases or when interventional treatment is not possible.
Endovascular treatment—balloon angioplasty and endografting
Balloon angioplasty has been used for the treatment of native and recurrent coarctation. While balloon angioplasty leads to a high rate of recurrent stenosis after native coarctation repair, it is the preferred treatment option for recurrent coarctation after previous surgical repair. The scar tissue at the site of the previous repair has a lower tendency for vascular remodeling, thus leading to a lower rate of aneurysmal dilation after angioplasty. Aortic wall injury occurs in approximately 1% to 2% of cases (83,84). The tendency towards restenosis seems to be lower than for native coarctation as well. Multiple studies have demonstrated the sound outcome of balloon angioplasty for restenosis (83,85-91). Balloon angioplasty for restenosis has been performed in children and infants with a high procedural success rate and a lower complication rate compared to open surgical repair (67,92). The reported procedural success rate ranges from 80% to nearly 100% (59,60,84,93). Freedom from restenosis after balloon angioplasty is quite variable, with reported rates of 6% to 53% (83,84). When restenosis occurs, simple re-dilation often leads to acceptable results (19).
Stent graft placement is an alternative to balloon angioplasty. This technique is newer than balloon angioplasty and has been first performed for native coarctation in 1991 (94). Over time, the indications for endovascular stent graft placement have broadened, and it has been used for the treatment of recurrent stenosis as well. Numerous case reports and studies have shown the feasibility and safety of TEVAR for re-coarctation (59,60,91,95,96). The procedural success rate ranges from 94% (97) to 97% (98). A significant reduction of the peak systolic gradient has been described with this technique: Hamdan et al. reported a reduction of the mean peak systolic gradient from 32±12 to 4±11 mmHg (97). In cases of an inadequate proximal landing zone, a pre-operative left carotid-subclavian bypass can be performed to optimize TEVAR placement.
However, complications can occur and have been reported in up to 18% (96). Complications include retroperitoneal bleeding (3%), device embolization (3%), aortic dissection or rupture (1–5%), stent migration (6%), coronary artery injury with subsequent myocardial infarction, postinterventional formation of arteriovenous fistulas (3%) and unplanned occlusion of the left subclavian artery that may necessitate post-interventional left carotid-subclavian bypass (52,59-61,91,97,99,100).
TEVAR is an appealing option for aneurysmal degeneration of aorta after previous coarctation repair as balloon angioplasty is not applicable. Reports and studies have shown a procedural success rate of up to 100% (101-105). Potential complications are similar to those caused by balloon angioplasty or stent graft placement for re-coarctation as described earlier. Endoleaks can occur due to suboptimal endograft apposition to the aortic wall, especially in cases of challenging anatomy. Another devastating complication that can occur in up to 10% of cases after stent graft implantation is spinal cord ischemia, leading to paraparesis or paraplegia (106). The usage of a cerebrospinal fluid drainage to monitor and regulate the cerebrospinal pressure has been shown to reduce the incidence of spinal cord injury (107). A higher mean arterial pressure is maintained to optimize cerebral perfusion pressure.
Surgical repair
When endovascular treatment is not successful or applicable, surgical repair remains the only treatment option left. Persistent endoleak or prosthetic infection after stent graft placement may also mandate surgical revision. The mortality rate is higher than for native coarctation repair and ranges from 1% to 3%, but can be as high as 5% to 10% for patients with significant comorbidities.
The most commonly used technique is the replacement of the re-stenosed segment with a straight tube graft via a left lateral thoracotomy. Single lung ventilation of the right lung can facilitate the dissection of the situs, which is usually complicated by adhesions from the previous operation. If the adhesions are dense and significant bleeding is encountered, the operation can be staged, where by the dissection is completed at the initial operation and the chest is temporarily closed after placement of chest drains and the patient is brought to the ICU for interim care. The patient is then returned to the operating room once the bleeding has stopped in 24–48 hours, for completing the reconstruction.
The “clamp and sew” technique has largely been abandoned in favor of usage of extracorporeal circulation to provide lower body perfusion to reduce the risks associated with lower body ischemia during the period of aortic clamping. Distal arterial access can be obtained either via the distal aorta or the left femoral artery. Venous drainage can be established either by the femoral vein or alternatively via the left atrium (through left inferior pulmonary vein) for left heart bypass. The cardiopulmonary bypass pump supplies the lower body through the distal arterial cannula, while the supra-aortic vessels are being perfused by the heart. The flow is titrated to maintain adequate perfusion in both circulatory beds. Once the aorta is proximally and distally clamped, the re-coarctated segment can be resected and replaced with a prosthetic graft.
In case of a challenging anatomy, the usage of hypothermic circulatory arrest can be helpful, allowing for an open proximal anastomosis. For hypothermic circulatory arrest, full cardiopulmonary bypass is established via arterial access as described above and right atrial cannulation via the femoral vein. As the patient is cooled and the heart begins to fibrillate, attention must be paid to ensure that left ventricle is not distended. This is generally not a problem in cases of little or no aortic regurgitation. If the LV is noted to be distended on TEE, a LV vent can be inserted via the left inferior pulmonary vein. Cardioplegia is not necessary for circulatory arrest periods less than 30 minutes. After the proximal anastomosis has been completed, the graft can be cannulated and circulation resumed to the heart and the supra-aortic branches through the graft. The distal anastomosis can then be performed under partial cardiopulmonary bypass. The advantage of hypothermic circulatory arrest for performing the operation is the minimal need for proximal dissection prior to ceasing of circulation. This dissection can be performed very easily during circulatory arrest after the aorta is opened. It also obviates the need for circumferential control of the aorta or any supra-aortic branches.
Open surgical repair as a redo operation is relatively safe, however, perioperative complications can occur. Potential complications directly related to surgery and often aggravated by dense adhesions include re-thoracotomy for bleeding (2%), pulmonary-associated complications included pneumothorax and pleural effusion (8%), injury of the laryngeal and the phrenic nerve (0%) as well as trauma of the esophagus or the thoracic duct (0–4%) (108,109). Other potential complications include lower body ischemia and spinal cord trauma with subsequent paralysis. Like for TEVAR, preoperative insertion of a cerebrospinal fluid drainage can help to reduce these risks (107). Distal aortic perfusion, hypothermia and neuro-monitoring including somatosensory and motor evoked potentials have been used by some centers to guide intraoperative hemodynamic management. Additionally, pattern of SSEP and MEP signals in the operating can also help set blood pressure goals to be maintained postoperatively in the ICU.
An alternative surgical approach is extra-anatomic bypass. This technique is usually not employed as a primary operation for children, but may be considered for complicated redo cases in older children or adults. The formation of dense adhesion after previous operations of the descending aorta can make redo - operation at this site very challenging. Extra-anatomic bypass offers the advantage of leaving these structures untouched by rerouting blood flow to the aorta distal to the stenosed segment. Several rerouting approaches have been suggested by different groups.
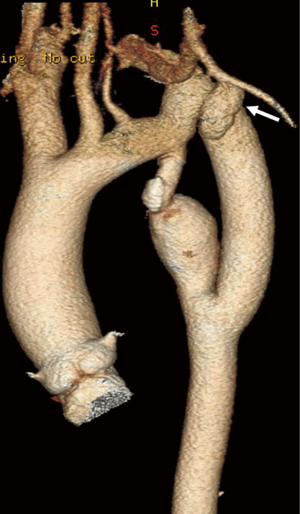
Extra-anatomic bypass from the left subclavian artery to the descending aorta (Figure 2) has been described as a feasible and safe treatment option for recurrent coarctation. Grinda and coworkers published a series of 16 patients who underwent extra-anatomic bypass grafting for complex aortic coarctation (46). This operation is performed via left posterolateral thoracotomy with left lung isolation. The dissection is limited to the area of the two anticipated anastomoses. A Dacron graft is anastomosed in an end to side fashion to the left subclavian artery, but can also be performed on the transverse aortic arch in the case of involvement of the subclavian artery. Distal anastomosis is performed on the descending thoracic aorta distal to the pathological segment in an end-to-side fashion. Both anastomoses can be performed under partial aortic cross-clamping, using a continuous 4-0 or 5-0 polypropylene suture without any need for cardiopulmonary bypass. The size of the Dacron prosthesis that is used is dependent on the size of the left subclavian artery, generally a 14 or 16 mm graft is selected. In series by Grinda et al, four patients were redo cases, while the remaining 12 patients had atypical anatomic coarctation. The peri-operative mortality in this series was 0%. No peri-operative complications were observed. During follow up, one patient died 10 years later due to non-surgery related reasons, and one patient required redo surgery.
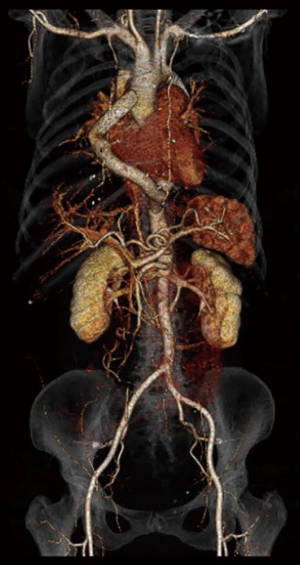
Another extra-anatomic approach is bypass grafting from the ascending to the descending aorta (Figure 3) (110) and has been suggested for complex redo cases. This operation is performed through a median sternotomy approach, thus avoiding the adhesions in the left pleural space. Cardiopulmonary bypass is initiated via aortic and venous cannulation. The heart is retracted cephalad and a longitudinal incision is made in the posterior pericardium to expose the descending aorta. A partial clamp is applied to the descending aorta and the distal anastomosis between the Dacron prosthesis and the descending aorta is performed in an end to side fashion using continuous 4-0 polypropylene suture. The Dacron prosthesis is routed posterior to the inferior vena cava, and around the right atrium to the ascending aorta. The proximal side-to-end anastomosis between the ascending aorta and the graft is done under partial side-clamping using a continuous 4-0 polypropylene suture. Prosthetic graft sizes commonly used range from 20 to 24 mm. Concomitant cardiac procedures can be performed as needed. Connolly and colleagues published a series of 18 patients who underwent this procedure. Most of the patients were redo cases, and the peri-operative mortality was 0%. Delmo Walter and coworkers reported similar results (111). Table 2 provides a summary of the various treatment options for recurrent coarctation and associated complication rates.
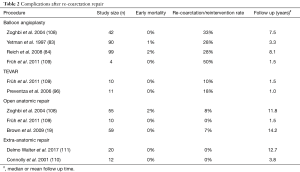
Full table
Conclusions
The surgical treatment of aortic coarctation has evolved over time, including both new surgical approaches and advanced perioperative management. This has led to a significant improvement of the short- and long-term outcome with lower recurrence rates for re-coarctation. However, recurrent coarctation remains a therapeutic challenge. The endovascular techniques for aortic pathologies have also been applied successfully to native and recurrent coarctation. However, some patients may not be suitable for endoluminal approaches and must be treated with open surgery using either in-situ reconstruction or extra-anatomic bypass. Given this, open surgical repair remains an important cornerstone in the therapeutic management of aortic re-coarctation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Morgagni GB. De sedibus et causis morborum. Epist 1760;XVIII:6.
- Ho SY, Anderson RH. Coarctation, tubular hypoplasia, and the ductus arteriosus. Histological study of 35 specimens. Br Heart J 1979;41:268-74. [Crossref] [PubMed]
- Price TP, Whisenhunt AK, Policha A, et al. Middle aortic coarctation. Ann Vasc Surg 2014;28:1314.e15-21. [Crossref] [PubMed]
- Mullen MJ. Coarctation of the aorta in adults: do we need surgeons? Heart 2003;89:3-5. [Crossref] [PubMed]
- Anderson RH, Lenox CC, Zuberbuhler JR. Morphology of ventricular septal defect associated with coarctation of aorta. Br Heart J 1983;50:176-81. [Crossref] [PubMed]
- Shinebourne EA, Tam AS, Elseed AM, et al. Coarctation of the aorta in infancy and childhood. Br Heart J 1976;38:375-80. [Crossref] [PubMed]
- Shone JD, Sellers RD, Anderson RC, et al. The developmental complex of “parachute mitral valve,” supravalvular ring of left atrium, subaortic stenosis, and coarctation of aorta. Am J Cardiol 1963;11:714-25. [Crossref] [PubMed]
- Warnes CA. Bicuspid aortic valve and coarctation: two villains part of a diffuse problem. Heart 2003;89:965-6. [Crossref] [PubMed]
- Becker AE, Becker MJ, Edwards JE. Anomalies associated with coarctation of aorta: particular reference to infancy. Circulation 1970;41:1067-75. [Crossref] [PubMed]
- Pádua LM, Garcia LC, Rubira CJ, et al. Stent placement versus surgery for coarctation of the thoracic aorta. Cochrane Database Syst Rev 2012;5:CD008204. [PubMed]
- Ringel RE, Gauvreau K, Moses H, et al. Coarctation of the Aorta Stent Trial (COAST): study design and rationale. Am Heart J 2012;164:7-13. [Crossref] [PubMed]
- Campbell M. Natural history of coarctation of the aorta. Br Heart J 1970;32:633-40. [Crossref] [PubMed]
- Crafoord C, Nylin G. Congenital coarctation of the aorta and its surgical treatment. J Thorac Cardiovasc Surg 1945;14:347-61.
- Gross R, Hufnagel C. Coarctation of the aorta. Experimental studies regarding its surgical correction. N Engl J Med 1945;233:287-93. [Crossref]
- Williams WG, Shindo G, Trusler G, et al. Results of repair of coarctation of the aorta during infancy. J Thorac Cardiovasc Surg 1980;79:603-8. [PubMed]
- Kappetein AP, Zwinderman A, Bogers A, et al. More than thirty-five years of coarctation repair. An unexpected high relapse rate. J Thorac Cardiovasc Surg 1994;107:87-95. [PubMed]
- Hesslein PS, McNamara D, Morriss M, et al. Comparison of resection versus partch aortoplasty for repair of coarctation in infants and children. Circulation 1981;64:164-8. [Crossref] [PubMed]
- Ziemer G, Jonas R, Perry S, et al. Surgery for coarctation of the aorta in the neonate. Circulation 1986;74:I25-31. [PubMed]
- Brown JW, Ruzmetov M, Hoyer MH, et al. Recurrent Coarctation: Is Surgical Repair of Recurrent Coarctation of the Aorta Safe and Effective? Ann Thorac Surg 2009;88:1923-30;discussion 1930-1.
- Dodge-Khatami A, Backer CL, Mavroudis C. Risk Factors for Recoarctation and Results of Reoperation: A 40-Year Review. J Card Surg 2000;15:369-77. [Crossref] [PubMed]
- Cramer JW, Ginde S, Bartz PJ, et al. Aortic aneurysms remain a significant source of morbidity and mortality after use of Dacron(®) patch aortoplasty to repair coarctation of the aorta: results from a single center. Pediatr Cardiol 2013;34:296-301. [Crossref] [PubMed]
- von Kodolitsch Y, Aydin MA, Koschyk DH, et al. Predictors of aneurysmal formation after surgical correction of aortic coarctation. J Am Coll Cardiol 2002;39:617-24. [Crossref] [PubMed]
- Bromberg BI, Beekman RH, Rocchini AP. Aortic aneurysm after patch angioplasty repair of coarctation: a prospective analysis of prevalence, screening tests and risks. J Am Coll Cardiol 1989;14:734-41. [Crossref] [PubMed]
- Knyshov GV, Sitar LL, Glagola MD, et al. Aortic aneurysms at the site of the repair coarctation of the aorta: a review of 48 patients. Ann Thorac Surg 1996;61:935-9. [Crossref] [PubMed]
- Parks WJ, Ngo TD, Plauth WH Jr, et al. Incidence of aneu-rysm formation after Dacron patch angioplasty repair for coarctation of the aorta: long term results and assessment utilizing magnetic resonance angiography with three dimen- sional surface rendering. J Am Coll Cardiol 1995;26:266-71. [Crossref] [PubMed]
- Ala-Kulju K, Heikkinen L. Aneurysm after patch graft aor- toplasty for coarctation of the aorta: long term results of surgical management. Ann Thorac Surg 1989;47:853-6. [Crossref] [PubMed]
- Vossschulte K. Isthmusplastik zur Behandlung der Aortenisthmus- stenose. Thoraxchirurgie 1957;4:443-50. [PubMed]
- Venturini A, Perna A, Bianchi G. Repair of coarctation of the tho- racic aorta without resection. Patch graft aortoplasty. Follow-up study of 46 cases. J Cardiovasc Surg (Torino) 1978;19:49-54. [PubMed]
- Walhout RJ, Lekkerkerker J, Oron G, et al. Comparison of polytetrafluoroethylene patch aorto- plasty and end-to-end anastomosis for coarctation of the aorta. J Thorac Cardiovasc Surg 2003;126:521-8. [Crossref] [PubMed]
- Waldhausen JA, Nahrwold D. Repair of coarctation of the aorta with a subclavian flap. J Thorac Cardiovasc Surg 1966;51:532-3. [PubMed]
- Pandey R, Jackson M, Ajab S, et al. Subclavian flap repair: review of 399 patients at median follow-up of fourteen years. Ann Thorac Surg 2006;81:1420-8. [Crossref] [PubMed]
- Barreiro CJ, Ellison TA, Williams JA, et al. Subclavian flap aortoplasty: still a safe, reproducible, and effective treatment for infant coarctation. Eur J Cardiothorac Surg 2007;31:649-53. [Crossref] [PubMed]
- Beekman RH, Rocchini A, Behrendt D, et al. Long-term outcome after repair of coarctation in infancy: Subclavian angioplasty does not reduce the need for reoperation. J Am Coll Cardiol 1986;8:1406-11. [Crossref] [PubMed]
- Amato JJ, Rheinlander H, Cleveland R. A method of enlarging the distal transverse arch in infants with hypoplasia and coarctation of the aorta. Ann Thorac Surg 1977;23:261-3. [Crossref] [PubMed]
- Thomson JDR, Mulpur A, Guerrero R, et al. Outcome after extended arch repair for aortic coarctation. Heart 2006;92:90-4. [Crossref] [PubMed]
- Hager A, Schreiber C, Nutzl S, et al. Mortality and restenosis rate of surgical coarctation repair in infancy: a study of 191 pa- tients. Cardiology 2009;112:36-41. [Crossref] [PubMed]
- Burch PT, Cowley CG, Holubkov R, et al. Coarctation repair in neonates and young infants: is small size or low weight still a risk factor? J Thorac Cardiovasc Surg 2009;138:547-52. [Crossref] [PubMed]
- Tabbutt S, Nicolson SC, Dominguez TE, et al. Perioperative course in 118 infants and children undergoing coarctation repair via a thoracotomy: a prospective, multicenter experience. J Thorac Cardiovasc Surg 2008;136:1229-36. [Crossref] [PubMed]
- Wright GE, Nowak CA, Goldberg CS, et al. Extended resection and end-to-end anastomosis for aortic coarctation in infants: results of a tailored surgical approach. Ann Thorac Surg 2005;80:1453-9. [Crossref] [PubMed]
- Kumar TK, Zurakowski D, Sharma R, et al. Prediction of recurrent coarctation by early postoperative blood pressure gradient. J Thorac Cardiovasc Surg 2011;142:1130-6, 1136.e1.
- Gross RE. Treatment of certain aortic coarctations by homologous grafts;a report of nineteen cases. Ann Surg 1951;134:753-68. [Crossref] [PubMed]
- Yousif A, Kloppenburg G, Morshuis WJ, et al. Repair of adult aortic coarctation by resection and interposition grafting. Interact Cardiovasc Thorac Surg 2016;23:526-30. [Crossref] [PubMed]
- Charlton-Ouw KM, Codreanu ME, Leake SS, et al. Open repair of adult aortic coarctation mostly by a resection and graft replacement technique. J Vasc Surg 2015;61:66-72. [Crossref] [PubMed]
- Heinemann MK, Ziemer G, Wahlers T, et al. Extraanatomic thoracic aortic bypass grafts: indications, techniques, and results. Eur J Cardiothorac Surg 1997;11:169-75. [Crossref] [PubMed]
- Almeida de Oliveira S, Lisboa LA, Dallan LA, et al. Extraanatomic aortic bypass for repair of aortic arch coarcta- tion via sternotomy: midterm clinical and magnetic resonance imaging results. Ann Thorac Surg 2003;76:1962-6. [Crossref] [PubMed]
- Grinda JM, Mace L, Dervanian P, et al. Bypass graft for complex forms of isthmic aortic coarctation in adults. Ann Thorac Surg 1995;60:1299-302. [Crossref] [PubMed]
- Bartoccioni S, Giombolini C, Fiaschini P, et al. Aortic coarctation, aortic valvular stenosis, and coronary artery disease: combined one-stage surgical therapy operation. J Card Surg 1995;10:594-6. [Crossref] [PubMed]
- Singer MI, Rowen M, Dorsey TJ. Transluminal aortic balloon angioplasty for coarctation of the aorta in the newborn. Am Heart J 1982;103:131-2. [Crossref] [PubMed]
- Rothman A, Galindo A, Evans WN, et al. Effectiveness and safety of balloon dilation of native aortic coarcta- tion in premature neonates weighing < or = 2,500 grams. Am J Cardiol 2010;105:1176-80. [Crossref] [PubMed]
- Suárez de Lezo J, Pan M, Romero M. Percutaneous interventions on severe coarctation of the aorta: a 21-year experience. Pediatr Cardiol 2005;26:176-89. [Crossref] [PubMed]
- Rao PS, Jureidini S, Balfour I, et al. Severe aortic coarctation in infants less than 3 months: successful palliation by balloon angioplasty. J Invasive Cardiol 2003;15:202-8. [PubMed]
- Fiore AC, Fischer LK, Schwartz T, et al. Comparison of an- gioplasty and surgery for neonatal aortic coarctation. Ann Thorac Surg 2005;80:1659-64. [Crossref] [PubMed]
- Cowley CG, Orsmond GS, Feola P, et al. Long-term, randomized comparison of balloon angioplasty and surgery for native coarctation of the aorta in childhood. Circulation 2005;111:3453-6. [Crossref] [PubMed]
- Rodés-Cabau J, Miró J, Dancea A, et al. Comparison of surgical and transcatheter treatment for native coarctation of the aorta in pa- tients > or = 1 year old. The Quebec Native Coarctation of the Aorta study. Am Heart J 2007;154:186-92. [Crossref] [PubMed]
- Wong D, Benson LN, Van Arsdell GS, et al. Balloon angioplasty is preferred to surgery for aortic coarcta- tion. Cardiol Young 2008;18:79-88. [Crossref] [PubMed]
- Mohan UR, Danon S, Levi D, et al. Stent implantation for coarctation of the aorta in children & lt;30 kg. JACC Cardiovasc Interv 2009;2:877-83. [Crossref] [PubMed]
- Meadows J, Minahan M, McElhinney DB, et al. Intermediate Outcomes in the Prospective, Multicenter Coarctation of the Aorta Stent Trial (COAST). Circulation 2015;131:1656-64. [Crossref] [PubMed]
- Qureshi AM, McElhinney DB, Lock JE, et al. Acute and intermediate outcomes, and evaluation of injury to the aortic wall, as based on 15 years experience of implanting stents to treat aortic coarctation. Cardiol Young 2007;17:307-18. [Crossref] [PubMed]
- Forbes TJ, Garekar S, Amin Z, et al. Procedural results and acute complications in stenting native and recurrent coarctation of the aorta in patients over 4 years of age: a multi-institutional study. Catheter Cardiovasc Interv 2007;70:276-85. [Crossref] [PubMed]
- Forbes TJ, Moore P, Pedra C, et al. Intermediate follow-up follow- ing intravascular stenting for treatment of coarctation of the aorta. Catheter Cardiovasc Interv 2007;70:569-77. [Crossref] [PubMed]
- Holzer R, Qureshi S, Ghasemi A, et al. Stenting of aortic coarcta- tion: acute, intermediate, and long-term results of a prospective multi-institutional registry--Congenital Cardiovascular Interven- tional Study Consortium (CCISC). Catheter Cardiovasc Interv 2010;76:553-63. [Crossref] [PubMed]
- Forbes TJ, Kim DW, Du W, et al. Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the CCISC (Congenital Cardiovascular Interventional Study Consortium). J Am Coll Cardiol 2011;58:2664-74. [Crossref] [PubMed]
- Tanous D, Benson LN, Horlick EM. Coarctation of the aorta: evaluation and management. Curr Opin Cardiol 2009;24:509. [Crossref] [PubMed]
- Bruckheimer E, Birk E, Santiago R, et al. Coarctation of the aorta treated with the Advanta V12 large diameter stent: acute results. Catheter Cardiovasc Interv 2010;75:402. [PubMed]
- Chakrabarti S, Kenny D, Morgan G, et al. Balloon expandable stent implantation for native and recurrent coarctation of the aorta--prospective computed tomography assessment of stent integrity, aneurysm formation and stenosis relief. Heart 2010;96:1212. [Crossref] [PubMed]
- Sohrabi B, Jamshidi P, Yaghoubi A, et al. Comparison between covered and bare Cheatham-Platinum stents for endovascular treatment of patients with native post-ductal aortic coarctation: immediate and intermediate-term results. JACC Cardiovasc Interv 2014;7:416. [Crossref] [PubMed]
- Rao PS, Galal O, Smith PA, et al. Five- to nine-year follow-up results of balloon angioplasty of native aortic coarctation in infants and children. J Am Coll Cardiol 1996;27:462-70. [Crossref] [PubMed]
- Sadiq M, Rehman AU, Qureshi AU, et al. Covered Stents in the management of native coarctation of the aorta – intermediate and long term follow up. Catheter Cardiovasc Interv 2013;82:511-8. [PubMed]
- Rao PS, Thapar MK, Kutayli F, et al. Causes of recoarctation after balloon angioplasty of unoperated aortic coarctation. J Am Coll Cardiol 1989;13:109. [Crossref] [PubMed]
- Fletcher SE, Nihill MR, Grifka RG, et al. Balloon angioplasty of native coarctation of the aorta: midterm follow-up and prognostic factors. J Am Coll Cardiol 1995;25:730. [Crossref] [PubMed]
- Pourmoghadam KK, Velamoor G, Kneebone JM, et al. Changes in protein distribution of the aortic wall following balloon aortoplasty for coarctation. Am J Cardiol 2002;89:91. [Crossref] [PubMed]
- Isner JM, Donaldson RF, Fulton D, et al. Cystic medial necrosis in coarctation of the aorta: a potential factor contributing to adverse consequences observed after percutaneous balloon angioplasty of coarctation sites. Circulation 1987;75:689. [Crossref] [PubMed]
- Moodie DS. Aortic dissection and coarctation. Curr Opin Cardiol 1990;5:649. [Crossref] [PubMed]
- Parikh SR, Hurwitz RA, Hubbard JE, et al. Preoperative and postoperative "aneurysm" associated with coarctation of the aorta. J Am Coll Cardiol 1991;17:1367. [Crossref] [PubMed]
- Baumgartner H, Bonhoeffer P, De Groot NMS, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 2008;118:e714. [Crossref] [PubMed]
- Feltes TF, Bacha E, Beekman RH 3rd, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation 2011;123:2607. [Crossref] [PubMed]
- Silversides CK, Kiess M, Beauchesne L, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan's syndrome. Can J Cardiol 2010;26:e80. [Crossref] [PubMed]
- Nistri S, Sorbo MD, Marin M, et al. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart 1999;82:19. [Crossref] [PubMed]
- Hahn RT, Roman MJ, Mogtader AH, et al. Association of aortic dilation with regurgitant, stenotic and functionally normal bicuspid aortic valves. J Am Coll Cardiol 1992;19:283. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). European Heart Journal. 2014;35:2873-926. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Yetman AT, Nykanen D, McCrindle BW, et al. Balloon angioplasty of recurrent coarctation: A 12- year review. J Am Coll Cardiol 1997;30:811-6. [Crossref] [PubMed]
- Reich O, Tax P, Bartakova H, et al. Long-term (up to 20 years) results of percutaneous balloon angioplasty of recurrent aortic coarctation without use of stents. Eur Heart J 2008;29:2042-8. [Crossref] [PubMed]
- Hellenbrand WE, Allen HD, Golinko RJ, et al. Balloon angio- plasty for aortic recoarctation: results of Valvuloplasty and Angioplasty of Conge- nital Anomalies Registry. Am J Cardiol 1990;65:793-7. [Crossref] [PubMed]
- Hijazi ZM, Fahey JT, Kleinman CS, et al. Balloon angioplasty for recurrent coarctation of aorta. Immediate and long-term results. Circulation 1991;84:1150. [Crossref] [PubMed]
- Anjos R, Qureshi SA, Rosenthal E, et al. Determinants of hemodynamic results of balloon dilation of aortic recoarctation. Am J Cardiol 1992;69:665. [Crossref] [PubMed]
- Witsenburg M. Balloon angioplasty for aortic recoarctation in children: initial and follow up results and midterm effect on blood pressure. Br Heart J 1993;70:170. [Crossref] [PubMed]
- Magee AG, Brzezinska-Rajszys G, Qureshi SA, et al. Stent implantation for aortic coarctation and recoarctation. Heart 1999;82:600. [Crossref] [PubMed]
- Harrison DA, McLaughlin PR, Lazzam C, et al. Endovascular stents in the management of coarctation of the aorta in the adolescent and adult: one year follow up. Heart 2001;85:561-6. [Crossref] [PubMed]
- Kpodonu J, Ramaiah VG, Rodriguez-Lopez JA, et al. Endovascular Management of Recurrent Adult Coarctation of the Aorta. Ann Thorac Surg 2010;90:1716-20. [Crossref] [PubMed]
- Brown ML, Burkhart HM, Connolly HM, et al. Late outcomes of reintervention on the descending aorta after repair of aortic coarctation. Circulation 2010;122:S81-4. [Crossref] [PubMed]
- Saxena A. Recurrent coarctation: interventional techniques and results. World J Pediatr Congenit Heart Surg 2015;6:257-65. [Crossref] [PubMed]
- O’Laughlin MP, Perry SB, Lock JE, et al. Use of endovascular stents in congenital heart disease. Circulation 1991;83:1923-39. [Crossref] [PubMed]
- Rosenthal E, Qureshi S, Tynan M. Stent implantation for aortic recoarctation. Am Heart J 1995;129:1220-1. [Crossref] [PubMed]
- Preventza O, Wheatley GH, Williams J, et al. Endovascular approaches for complex form of recurrent aortic coarctation. J Endovasc Ther 2006;13:400-5. [Crossref] [PubMed]
- Hamdan MA, Maheshwari S, Fahey JT, et al. Endovascular stents for coarctation of the aorta: initial re- sults and intermediate-term follow-up. J Am Coll Cardiol 2001;38:1518-23. [Crossref] [PubMed]
- Marshall AC, Perry SB, Keane JF, et al. Early results and medium-term follow-up of stent implantation for mild re-sidual or recurrent aortic coarctation. Am Heart J 2000;139:1054-60. [Crossref] [PubMed]
- Walhout RJ, Lekkerkerker JC, Oron GH, et al. Comparison of surgical repair with balloon angioplasty for native coarc- tation in patients from 3 months to 16 years of age. Eur J Cardiothorac Surg 2004;25:722-7. [Crossref] [PubMed]
- Treacy EP, Duncan WJ, Tyrell MJ, et al. Neurological complications of balloon angioplasty in children. Pediatr Cardiol 1991;12:98-101. [Crossref] [PubMed]
- Parsa P, Eidt J, Rios A, et al. Case Report: An Innovative Endovascular Technique for Repair of Descending Thoracic Aortic Aneurysm following an Open Coarctation Repair. Ann Vasc Surg 2018;46:205.e1-205.e4. [Crossref] [PubMed]
- Bell RE, Taylor PR, Aukett M, et al. Endoluminal Repair of Aneurysms Associated With Coarctation. Ann Thorac Surg 2003;75:530-3. [Crossref] [PubMed]
- Gawenda M, Aleksic M, Heckenkamp J, et al. Endovascular repair of aneurysm after previous surgical coarctation repair. J Thorac Cardiovasc Surg 2005;130:1039-43. [Crossref] [PubMed]
- Hörmann M, Pavlidis D, Brunkwall J, et al. Long-term results of endovascular aortic repair for thoracic pseudoaneurysms after previous surgical coarctation repair. Interact Cardiovasc Thorac Surg 2011;13:401-4. [Crossref] [PubMed]
- Perera AH, Rudarakanchana N, Hamady M, et al. New-generation stent grafts for endovascular management of thoracic pseudoaneurysms after aortic coarctation repair. J Vasc Surg 2014;60:330-6. [Crossref] [PubMed]
- Scott DA, Denton MJ. Spinal cord protection in aortic endovascular surgery. Br J Anaesth 2016;117 Suppl 2:ii26-31. [Crossref] [PubMed]
- Ullery BW, Cheung AT, Fairman RM, et al. Risk factors, outcomes, and clinical manifestations of spinal cord ischemia following thoracic endovascular aortic repair. J Vasc Surg 2011;54:677-84. [Crossref] [PubMed]
- Zoghbi J, Serraf A, Mohammadi S, et al. Is surgical inter- vention still indicated in recurrent aortic arch obstruction? J Thorac Cardiovasc Surg 2004;127:203-12. [Crossref] [PubMed]
- Früh S, Knirsch W, Dodge-Khatami A, et al. Conparison of surgical and interventional therapy of native and recurrent aortic coarctation regarding different age groups during childhood. Eur J Cardiothorac Surg 2011;39:898-904. [Crossref] [PubMed]
- Connolly HM, Schaff HV, Izhar U, et al. Posterior Pericardial Ascending-to-Descending Aortic Bypass An Alternative Surgical Approach for Complex Coarctation of the Aorta. Circulation 2001;104:I133-7. [Crossref] [PubMed]
- Delmo Walter EM, del Maria Javier M, Hetzer R. Extra-anatomical bypass in complex and recurrent aortic coarctation and hypoplastic arch. Interact Cardiovasc Thorac Surg 2017;25:400-6. [Crossref] [PubMed]
Cite this article as: Beckmann E, Jassar AS. Coarctation repair—redo challenges in the adults: what to do? J Vis Surg 2018;4:76.


