Intimal re-layering technique for type A acute aortic dissection—reconstructing the intimal layer continuity to induce remodeling of the false channel
Introduction
Type A acute aortic dissection (TAAAD) represents a clinical catastrophe. Operative mortality during the last decades has reduced due to the progress of perioperative management. A widespread awareness of aortic pathology as well as the progress of imaging technologies of operative and post-operative treatment has radically improved the results of acute surgery also in high risk and extremely fragile patient (1).
The goals of surgery are to save life by prevention of pericardial tamponade or intra-pericardial aortic rupture, to resect the primary entry tear, to correct or prevent any malperfusion and aortic valve regurgitation, and if possible to prevent late dissection-related complications in the proximal and downstream aorta (2). Hemiarch replacement is the procedure of choice unless arch tears impose a more radical treatment.
The fate of aortic dissection patients depends on the evolution of the false channel.
It has been widely demonstrated that the patency of the false channel determines the long term survival and freedom from re-operation (3-5).
Based upon these considerations, recently many authors advocate the choice of more radical operations, with the replacement of the aortic arch and the use of frozen elephant trunks to exclude the false channel since the initial operation (6-8).
The advantages of this approach, for a number of reasons, are not widely accepted (9-12). These include: a higher surgical risk, the availability, in the acute phase, of surgeons specialized in arch surgery, the real clinical advantage of an immediate radical correction compared to an elective re-operation of selected patients requiring a treatment of the residual thoracic aorta (13-15).
However after initial operation these patients have an advantage from arch replacement with better evolution and remodeling of the false channel (6-9).
Hence we analyzed our recent series of TAAAD patients to identify the factors and the anatomical features of flow circuits influencing the patency of false channel after standard hemiarch replacement.
Starting from these data and from the necessity to find a balance between a more efficient initial operation and overall risk, while maintaining an acceptable complexity and a good reproducibility of current hemiarch treatment, we designed the approach described herein.
Background studies
Preoperative and postoperative contrast CT scans of 191 out of 303 patients—operated in Siena between September 2006 and June 2017 of TAAAD using standard hemiarch technique—were reviewed by a surgeon and a vascular radiologist to assess the anatomy of intimal flap at presentation, to analyze the persistence of a false channel and the location of intimal entry tears after repair.
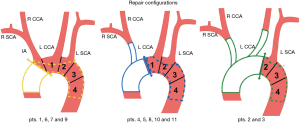
Accordingly false channel perfusion was classified as follows: (I) flow maintained by a tear in the arch intima; (II) by a tear located in supra-aortic branches; (III) by a tear in the aorta distal to the left subclavian artery and (IV) no perfusion.
The absence of a residual dissection was observed in 58 patients (30.4%) while a patent false channel was present in 132 patients (69.1%). Type 2 DeBakey presentation occurred in 45 patients: all of these (100%) had not a postoperative false channel. In the remaining 146 patients, all with a DeBakey Type1 presentation, 90.4% (132 patients) had a patent false channel after surgical repair. Among these 132 patients with residual dissection 33 (25%) had a tear at the level of the arch intima; 77 patients (58.3%) had an entry tear located at the level of one of supra aortic trunks and in 22 (16.7%) the false channel was perfused from an entry tear in the distal aorta (Figure 1A).
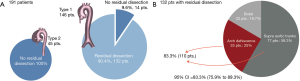
Entry tears from the arch and supra aortic trunks totaled 83.3% (95% CI: 75.9–89.3%) (Figure 1B).
These data indicate clearly that entry tears in the arch area represent the cause of a perfused false channel in the majority of cases of TAAAD treated with hemiarch replacement. This data can be regarded as a support to total arch replacement strategy, nevertheless it represents also the rationale for the intimal re-layering technique. This concept will be expanded in the discussion.
Methods
Between August 2016 and January 2018, 39 patients with TAAAD were operated in Siena University Hospital within 24h from the onset; of these, 11 patients underwent emergency surgery using the new technique called Intimal re-layering. Clinical characteristics of the patient population are summarized in the Table 1.
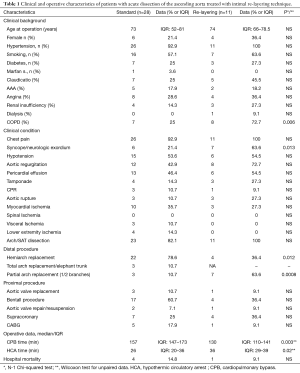
Full table
This retrospective study did not require approval by an ethical committee. Patient identification remained anonymous and informed consent was waived due to the observational nature of the study. Requirement for informed patient consent was waived because of the emergency setting.
Operative technique
All operations in this series were performed through a median sternotomy. In aortic dissection procedures, an arterial line with a side branch is routinely set up, for alternative arterial cannulation sites. Arterial pressure monitor line is placed on left radial artery. Intraoperative trans-esophageal echocardiography (TEE) is routinely used in type A aortic dissection procedures. Nasopharyngeal, esophageal, and bladder temperatures are monitored. Cerebral oxygenation is monitored in all patients by measuring rSO2 values derived from an INVOS 5100C cerebral oximeter (INVOS, Covidien, Mansfield, MA, USA).
Right axillary artery is the preferential site for cannulation and it was used for all procedures. Direct cannulation using longitudinal arteriotomy is our preferred method of axillary cannulation; Edwards OptiSiteTM cannula (Edwards Lifesciences, Irvine, CA, USA) are routinely used.
In all cases, moderate hypothermic circulatory arrest was a planned procedure to allow resection by means of an open technique (Figure 2).

Cardiopulmonary bypass flows, with cardiac indices of 2.0 to 2.5 L/min/m2, is maintained. Cooling is considered to be adequate for hypothermic systemic circulatory arrest when the nasopharyngeal temperature has reached 25 °C, and the bladder temperature is below 26 °C.
During cooling the aortic arch is prepared, exposing the supra aortic trunks and distally beyond the origin of the left subclavian artery; care is taken to mobilize the left laryngeal nerve. At the desired temperature, the supra aortic trunks are clamped table is tilted down and the systemic arterial flow is then totally arrested. Right brachiocephalic perfusion is maintained until the aorta is opened and a perfusion cannula is inserted into the common carotid artery. Blood cardioplegia is administered intermittently retrogradely through the coronary sinus. The ascending aorta is opened in a standard fashion and the arch inspected for intimal tears. The hemiarch is prepared with generous resection in the concavity of the aorta. Resection can be extended to zone 1 or 2 if the presence of tears at the level of the ostia or the quality of the trunks recommend their replacement. Once obtained the level of resection intimal re-layering is performed. A tubular Dacron or PTFE graft of adequate size is gently inserted into the aortic arch and the proximal aorta, thus creating an elephant trunk (Figure 3A) Care must be taken to avoid any intimal damage. In this phase a perfect vision of the distal arch and proximal descending aorta is crucial therefore strong suction into the open aorta is applied.
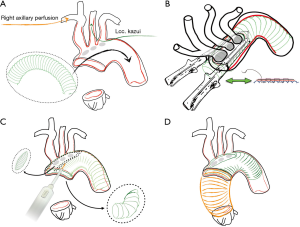
This graft represents the new intimal layer, it should not exceed the diameter of the true lumen. The length of the graft should not exceed 10–15 cm: too long grafts are difficult to advance, however their length should be sufficient to effectively redirect blood into the true channel. If longer grafts are sought Amplatz Goose Neck Snare (Microvena, White Bear Lake, MN, USA), advanced in the true lumen under radiological or TEE vision, is used to pull the graft into the descending aorta. Once obtained the optimal positioning of the graft into the arch a continuous suture (4.0 prolene with external Teflon reinforcement) is performed around the island of SAT thus securing the graft to the full thickness of the layers of the dissected aorta. To speed up this passage a bladeless linear stapler (DST Series™ GIA™ 60 mm Single Use Knifeless Stapler, Covidien Medtronic, Minneapolis, USA) was used in the latest patients with optimal quality of suturing (Figure 3B). The graft is then fenestrated with cautery to provide flow to arch vessels and the excess of the graft is trimmed along the edge of the aorta (hemiarch) (Figure 3C).
Once proximal, the re-layering is completed a second tubular graft is anastomosed to the hemiarch.: this anastomosis may be reinforced externally with a Teflon strip, while the internal reinforcement is the “re-layering” graft itself. After de-airing the graft is clamped, the systemic perfusion is resumed and rewarming is begun. During rewarming, the aortic root portion of the procedure is completed (Figure 3D).
TEE was used to identify the flow in the true channel and the correct deployment of the elephant trunk portion of the repair.
Follow up
Ct scan follow-up was performed on all survivors. Angio-CT with late contrast phase is performed before hospital discharge or within the first month of operation (by protocol) unless renal dysfunction contraindicates it. Subsequent CT scans are performed at 6 months, 1 year and afterward every 12/18 months.
Statistical analysis
Continuous data are reported as median and interquartile range (25th–75th percentile); categorical variables are reported as counts and percentages. For comparison of continuous unequally distributed variables, the Wilcoxon test for unpaired data was employed. Categorical variables were compared using the N-1 Chi-squared test. All statistical calculations were performed using R {R Core Team [2014], Available at: http://www.R-project.org/}. For time to event data, survival analysis using Kaplan-Meier was carried out using JMP 8 (SAS Institute, Cary, NC, USA).
Results
All patients had a supra-coronary repair; one patient had aortic valve replacement + CABG. In the first two patients, a manual suture around supra-aortic trunks was used; the subsequent seven patients were treated with a mechanical suture bladeless device; a Dacron (nine patients) or PTFE graft (two patients) was used to reinforce intima, with size range between 18 and 24 mm (median 22 mm).
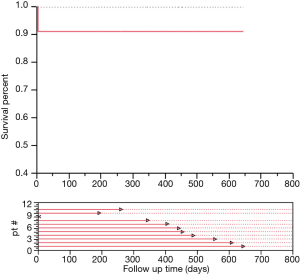
No patient died in the operating room; no neurologic deficit was observed upon awakening. Agitation characterized the postoperative course of on patient and resolved at discharge One patient died in POD 5th for low cardiac output syndrome. Median cardiopulmonary bypass time was 130 min (IQR, 110–141 min); median arrest time for re-layering was 17 min (IQR, 16–20 min); median total arrest was 36 min (IQR, 29–39 min) (Figure 4). Distal aortic anastomosis was performed in zone 0 in 4 patients, zone 1, with innominate replacement, in 5 patients, in zone 2, with branches to innominate and left common carotid arteries, in 2 patients (Figure 5). Median ICU stay was 3 days (IQR, 2–6 days); Hospital mean length of stay was 15.2±8 days. Median follow up (closing date 06/01/2018) was 443 days (IQR, 262–557 days); no late deaths occurred. Figure 6 illustrates the follow-up results.

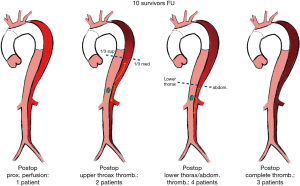
No dehiscence at the level of stapler or manual sutures was observed Proximal one-third of the thoracic aorta false channel was obliterated in all cases but one; in 3 cases complete exclusion of the false channel was obtained after the operation (Figure 7). In one case stent graft completion was required.
Discussion
In patients undergone acute TAAAD surgery long-term outcome is strongly dependent upon false lumen patency (3-5); the persistence of a circulating dissection even after repair is attributable to intimal discontinuities along the arch, arch vessels and descending aorta.
Therefore any intervention designed to improve intimal continuity by reducing intimal entries may improve the outcome of these patients. Based on these considerations many authors recommend a total arch replacement on a routine basis in TAAAD (7,8).
The intimal re-layering technique combines the advantages of arch replacement and elephant trunk technique with the simplicity of a standard hemiarch repair.
The procedure can be regarded as an adjunct to current hemiarch replacement and as a simplification of the island (en bloc) repair (17).
The goals are threefold: (I) to reinforce the intima at the arch level with a prosthetic layer; (II) to avoid flow between the false lumen around the arch vessels and that of the distal aorta; (III) to provide an elephant trunk configuration for further possible interventions in the distal aorta.
The use of a bladeless stapler reduced circulatory arrest time while providing an excellent suture line. The use of the stapler is off-label with regard to vascular anastomosis: this is the reason for we do not consider yet the re-layering technique our standard approach. It is used exclusively in fragile cases, requiring an arch treatment, but not suitable for standard arch replacement.
We use the stapler to perform a suture line that enables an “endo island”. Actually, it creates a suture line of consolidation of the dissected layers with the new intima. With the use of staplers we create two converging suture lines that end beyond the ostium of left subclavian artery: in most cases the space between the two edges of the suture lines is small, therefore no more than two U stitches (from inside out) are sufficient to obliterate the space and to give continuity to the suture of the supra-aortic trunks island.
The suture of the stapler is extremely resistant and delicate with the tissues: preliminary tests on explanted aortic dissection tissue and animal aorta show the resistance of such suture lines that are distributed on multiple lines.
We have not observed any dehiscence at imaging follow up. At necropsy, the only patient who died postoperatively did not show any tear at the level of the stapler stitches; the false channel appeared obliterated by organized thrombus in the proximal aorta, in the proximal left subclavian artery (2–3 cm) and between the two layers of the SAT island, where actual flow was suppressed.
Compared to standard arch replacement the re-layering technique has the advantage to be easy to learn and to perform: with this technique, also a less experienced surgeon may provide the patient with an Elephant trunk configuration with ease and in a short time, without disconnecting supra-aortic trunks that (especially the left subclavian artery) may be difficult to repair. Moreover, the prosthetic new intimal layer provides an overall reinforcement of the aortic wall, it does not only reinforce the anastomosis but also the arch intima, thus increasing the safety of the standard hemiarch replacement; in the meantime the ET configuration, by redirecting flow far from the arch area reduces the tension of the arch structures.
Actually, the right perspective to appraise this procedure is to compare it to the standard hemiarch repair rather than to arch replacement. Intimal re-layering, in addition to a hemiarch repair, provides the patient with a 10–15 cm “prosthetic channel”, which ends into the true lumen beyond the aortic isthmus. Moreover, reentries from supra-aortic trunks are kept separate from the “systemic” false channel, thus “cutting” some deleterious circuit which feeds the false lumen from the proximal aorta (Figure 8) As reported in the background section, we found that significant entries, maintaining the false channel in the descending thoracic aorta, originate from the Supra-Aortic trunks in 58% or from arch intimal tears in 25%. This indicates that more than two-thirds of those subjects could have taken advantage from an “intimal” correction at the arch level, or from the separation of the systemic aortic false channel from entries located into the arch vessels.
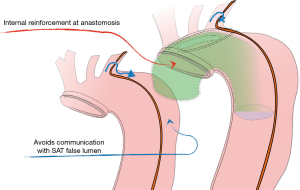
Conclusions
This technique combines the advantages of arch replacement to the simplicity of anterior hemiarch repair. This study demonstrates the safety of the procedure and the possibility to induce aortic remodeling without complex arch replacement.
In selected cases, this technique can be considered for other conditions, such as diffuse aortic disease or type B dissections, when an elephant trunk repair would be desirable without a complex arch replacement.
Intimal re-layering, especially with the use of dedicated stapling devices and grafts, may represent a new class of aortic interventions targeted to the intima.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study did not require approval by an ethical committee. Patient identification remained anonymous and informed consent was waived due to the observational nature of the study.
References
- Berretta P, Patel HJ, Gleason TG, et al. IRAD experience on surgical type A acute dissection patients: results and predictors of mortality. Ann Cardiothorac Surg 2016;5:346-51. [Crossref] [PubMed]
- Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol 2011;58:2455-74. [Crossref] [PubMed]
- Fattouch K, Sampognaro R, Navarra E, et al. Long-term results after repair of type a acute aortic dissection according to false lumen patency. Ann Thorac Surg 2009;88:1244-50. [Crossref] [PubMed]
- Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;133:127-35. [Crossref] [PubMed]
- Kimura N, Tanaka M, Kawahito K, et al. Influence of patent false lumen on long-term outcome after surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg 2008;136:1160-6, 1166.e1-3.
- Shrestha M, Fleissner F, Ius F, et al. Total aortic arch replacement with frozen elephant trunk in acute type A aortic dissections: are we pushing the limits too far? Eur J Cardiothorac Surg 2015;47:361-6; discussion 366. [Crossref] [PubMed]
- Sun L, Qi R, Zhu J, et al. Total arch replacement combined with stented elephant trunk implantation: a new "standard" therapy for type a dissection involving repair of the aortic arch? Circulation 2011;123:971-8. [Crossref] [PubMed]
- Uchida N, Katayama A, Tamura K, et al. Frozen elephant trunk technique and partial remodeling for acute type A aortic dissection. Eur J Cardiothorac Surg 2011;40:1066-71. [PubMed]
- Di Eusanio M, Berretta P, Cefarelli M, et al. Total Arch Replacement Versus More Conservative Management in Type A Acute Aortic Dissection. Ann Thorac Surg 2015;100:88-94. [Crossref] [PubMed]
- Westaby S, Saito S, Katsumata T. Acute type A dissection: conservative methods provide consistently low mortality. Ann Thorac Surg 2002;73:707-13. [Crossref] [PubMed]
- Kazui T, Washiyama N, Muhammad BA, et al. Extended total arch replacement for acute type a aortic dissection: experience with seventy patients. J Thorac Cardiovasc Surg 2000;119:558-65. [Crossref] [PubMed]
- Urbanski PP, Siebel A, Zacher M, et al. Is extended aortic replacement in acute type A dissection justifiable? Ann Thorac Surg 2003;75:525-9. [Crossref] [PubMed]
- Etz CD, Plestis KA, Homann TM, et al. Reoperative aortic root and transverse arch procedures: a comparison with contemporaneous primary operations. J Thorac Cardiovasc Surg 2008;136:860-7, 867.e1-3.
- Preventza O, Garcia A, Cooley DA, et al. Reoperations on the total aortic arch in 119 patients: short- and mid-term outcomes, focusing on composite adverse outcomes and survival analysis. J Thorac Cardiovasc Surg 2014;148:2967-72. [Crossref] [PubMed]
- Quintana E, Bajona P, Schaff HV, et al. Open aortic arch reconstruction after previous cardiac surgery: outcomes of 168 consecutive operations. J Thorac Cardiovasc Surg 2014;148:2944-50. [Crossref] [PubMed]
- Neri E, Tucci E, Tommasino G, et al. Using a median sternotomy and a deltopectoral incision for axillary artery cannulation, accurate exposure of arch vessels is performed. Asvide 2018;5:423. Available online: http://www.asvide.com/article/view/24388
- Di Eusanio M, Castrovinci S, Tian DH, et al. Antegrade stenting of the descending thoracic aorta during DeBakey type 1 acute aortic dissection repair. Eur J Cardiothorac Surg 2014;45:967-75. [Crossref] [PubMed]
Cite this article as: Neri E, Tucci E, Tommasino G, Guaccio G, Ricci C, Lucatelli P, Cini M, Ceresa R, Benvenuti A, Muzzi L. Intimal re-layering technique for type A acute aortic dissection—reconstructing the intimal layer continuity to induce remodeling of the false channel. J Vis Surg 2018;4:82.