Endoscopic Port AccessTM left ventricle outflow tract resection and atrioventricular valve surgery
Introduction
Adult hypertrophic obstructive cardiomyopathy (HOCM) is as an autosomal dominant cardiac sarcomere protein gene abnormality associated with an annual 1–2% sudden cardiac death risk (1) and is defined as unexplained left ventricular (LV) thickness greater than 15 mm in 1 or more LV segments resulting in LV outflow tract obstruction (LVOTO), LV-diastolic dysfunction and conduction abnormalities. Systolic anterior motion (SAM) and regurgitation of the mitral valve (MV) may occur due to concomitant anterior MV-leaflet elongation and anterior papillary muscle displacement towards the LV outflow tract (LVOT). Degenerative-, infective- and rheumatic atrioventricular valve (AVV) disease may also be present as independent pathology in the context of HOCM and LVOTO. Left ventricle septal myomectomy (LVSM) with or without concomitant AVV surgery is conventionally performed either by isolated trans-aortic, trans-atrial, trans-ventricular or combined trans-aortic valve and left atrial access (2-8) through midline sternotomy. The progressive paradigm shift towards endoscopic (9), robotic (10) and trans-catheter cardiac interventions (11,12) resulted in innovative techniques to simultaneously address LVOTO and AVV pathology. This brief report of 13 consecutive HOCM patients with concomitant AVV pathology, presents the perioperative and long-term clinical and echocardiographic outcomes of LVSM and AVV surgery by Endoscopic Port AccessTM Surgery (EPAS), operated by our current surgical team between March 1st 2010 and October 31st 2015.
Patient selection and work-up
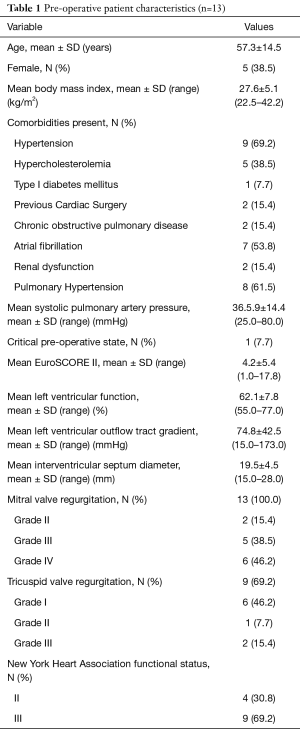
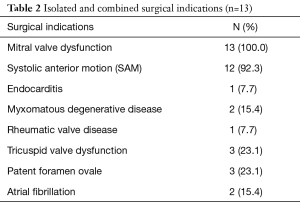
EPAS is the routine procedure for isolated AVV disease at our institution (13) and no pre-operative contra-indications prohibit our endoscopic approach. We reported our experience in difficult access extreme obese patients (14), congenital chest wall deformities (15), redo-cardiac surgery (16) and redo-EPAS after previous EPAS (17). The relevant preoperative HOCM patient characteristics and surgical indications are outlined in Tables 1,2 respectively. Presenting symptoms included angina, syncope and dyspnoea in 6 (46.2%), 4 (30.8%) and 9 (69.2%) patients respectively. The pre-operative peak LVOT gradient of 173 mmHg measured in one patient was confirmed by trans-oesophageal echocardiography (TEE) and catheterization. Two (15.4%) patients had previous cardiac operations, which included a minimally invasive direct coronary artery bypass grafting (104.3 months prior to current HOCM presentation) and conventional sternotomy aortic valve replacement with Morrow-procedure (1.3 months prior current HOCM presentation) at another institution.
Full table
Full table
Pre-operative preparation
Pre-operative aorta-iliac-femoral-axis (AIFA) evaluation is routinely performed in all patients either during coronary catheterization or by magnetic resonance angiography. We do not routinely perform computerized tomography in redo-surgery and perform no special investigation to evaluate the presence of lung adhesions. Routine cardiac surgical workup is followed by an elaborate informed consent process in which the various options are discussed and a final treatment strategy elected.
Equipment preference card
All LVSM and isolated AVV procedures in the context of HOCM are performed by EPAS using special long shafted instruments without additional special equipment. A bovine pericardial patch is used to augment the anterior MV leaflet if required.
Procedure
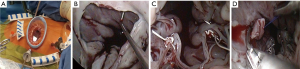
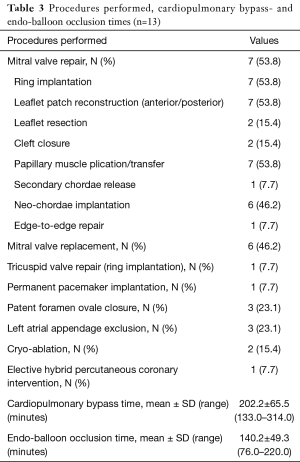
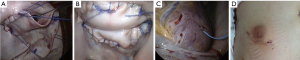
Our routine EPAS technique (18) is modified in the context of HOCM and concomitant AVV pathology. However, the standard EPAS operative setup remains unchanged (Figure 1A). Peripheral cardiopulmonary bypass (CPB) is established by TEE-guided cannulation of the right internal jugular vein (16–18 Fr, OptisiteTM, Edwards Lifesciences, Irvine, California, USA), right femoral vein (22–25 Fr, QuickdrawTM, Edwards Lifesciences, Irvine, California, USA) and right femoral artery cannulation (21 or 23 Fr, EndoreturnTM, Edwards Lifesciences, Irvine, California, USA). An endo-aortic balloon (IntraCludeTM, Edwards Lifesciences, Irvine, California, USA) is utilised for aortic occlusion and delivery of cold antegrade crystalloid cardioplegia. Routine and complex MV and tricuspid valve (TV) surgery, as outlined in Table 3, are performed with long shafted instruments through a 4th intercostal, antero-lateral thoracic working port. Access to the LVOT is obtained by detaching the anterior MV-leaflet from the annulus (Figure 1B), which is followed by routine plication of both papillary muscles away from the LVOT (Figure 1C). Controlled sharp LV-septal muscle resection is performed from the base of the aortic valve to the papillary muscles (Figure 1D), of which the excision depth is guided by pre-operative LV-septal diameter by TEE measurements. LV-septum perforation and extensive resection in conduction regions are avoided. In cases of MV-repair, an oversized bovine pericardium patch is incorporated into the annular re-attachment (Figure 2A,B). Significant SAM was present in 12 (92.3%) patients, of which successful MV repair was achieved in 7 (53.8%). MV-replacements were reserved for rheumatic, advanced myxomatous or high-risk valves for short-term repair failure. For TV surgery through a right atriotomy, the superior and inferior vena cave are snared. Cryo-ablation is performed with an argon-gas surgical ablation system (Medtronic, Minneapolis, USA), the base of the left atrial appendage oversewn in patients with atrial fibrillation or previous stroke and patent foramen ovale also routinely closed. Post-procedural de-airing is ensured by left atrial and aortic balloon venting catheters, continuous flooding of the operative field with CO2 and TEE surveillance for residual air in the left ventricle. Temporary epicardial (Figure 2C) or trans-jugular ventricular pacing wires are routinely placed.
Full table
Team members
All patients are presented at a specialised multi-disciplinary team meeting, which consist of cardiac surgical, cardiology, anaesthetic, radiology, allied medical, trainee and administrative staff members. Operative procedures are conducted within a specialised team context that consist of a TEE trained anaesthetist (19), two minimally invasive cardiac surgeons and a trainee, operative nurses and a perfusionist experienced in EPAS perfusion (20) procedures. Post-operative intensive care is coordinated by a team of full-time on-site cardiac intensivists, which is followed by a structured and individualised in-hospital multi-disciplinary rehabilitation program. Finally, continuation of care ascertained by the referring physician as part of patient centred service delivery.
Post-operative management
Cardio-respiratory support, sedation and analgesia are administered as indicated in intensive care and a structured in-hospital rehabilitation program (Figure 2D) initiated as soon as possible. All patients undergo pre-discharge trans-thoracic echocardiographic evaluation for satisfactory operative result confirmation. Infective endocarditis is treated with appropriate antibiotics for 6 weeks under the supervision of an infective endocarditis team and long-term anti-coagulation regimes initiated and stabilized in-hospital in cases of mechanical prosthetic implantation or chronic atrial fibrillation. All patients are reviewed within 6 weeks post-discharge, after which continuation of care is ascertained by the referring cardiologist and family physician.
Outcomes
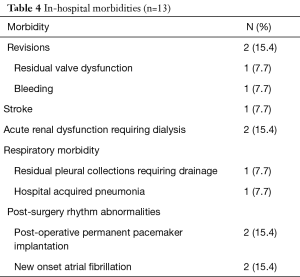
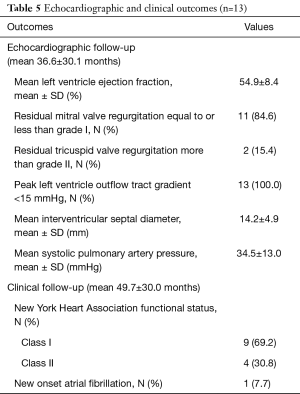
There were no sternotomy conversions, complications in establishing vascular or LVOT access, LV-septum perforations or 30-day mortalities. One (7.7%) patient presented with active staphylococcal MV-endocarditis, a peak instantaneous LVOT gradient of 88.0 mmHg and interventricular septum diameter (IVSD) of 22 mm. The patient underwent extensive anterior and posterior MV-leaflet resection, debridement, autologous annular patch and leaflet xenograft pericardial patch reconstruction in addition to LVOT resection. Revisions for bleeding (n=1, 7.7%) and residual MV-dysfunction post-repair (n=1, 7.7%) were performed through the same incisions without residual complications. One (7.7%) patient developed an ischemic stroke on the 10th post-operative day due to a MV-mechanical prosthesis thrombosis that resolved with conservative therapy. The patient was eventually discharged after 72 days in hospital. There were no wound infections or persistent air leaks and the mean length of hospitalization was 17.7±18.1 days (range, 7.0–72.0 days). Other in-hospital morbidities are outlined in Table 4. Post-operative dialysis was required in 2 (15.4%) patients, of whom 1 (7.7%) had severe renal impairment pre-operatively. Both patients were subjected to cardiopulmonary perfusion times of 133.0 minutes. Post-discharge echocardiographic and clinical data (645.7 patient-months) are described in Table 5 and were obtained by reviewing the latest consultation records (n=13, 100.0% complete), of which 12 (92.3%) patients had follow-up periods longer than 2 years. Clinical follow-up (mean, 49.7±30.0 months) for survival identified two late mortalities at 40.1 (fatal stroke, age 82.6 years) and 85.0 post-operative months (ischemic colitis, age 76.5 years) respectively. There were no procedure-, HOCM-, AVV-related late mortalities or re-interventions. All patients (n=13, 100.0%), including the late mortalities, were classified as New York Heart Association (NYHA) I or II during their latest clinical review. Echocardiographic follow-up (mean, 36.6±30.1 months) identified no residual or recurrent peak instantaneous LVOT gradients more than 15 mmHg. Asymptomatic chordal SAM was diagnosed in 1 (7.7%) patient with a mean gradient of 11 mmHg (40.1 months post-operative).
Full table
Full table
Tips, tricks and pitfalls
LVOT resection with or without AVV surgery is most commonly performed through conventional mid-line sternotomy and is not recommended as part of the initial EPAS skills acquisition in upcoming centres. For experienced EPAS operators, this approach provides direct and targeted access to the LVOT, the majority of the interventricular septum, the MV and TV. Plication of the papillary muscles away from the LVOT reduces the risk of residual valvular and chordal SAM and is considered an essential step for a successful LVOT gradient reduction outcome. Annular detachment of the anterior MV should be wide and extend from segment A1 to A3. Careful suture retraction of the detached anterior MV-leaflet facilitates unobstructed endoscopic access to the LVOT. LVSM depth is determined by pre-operative TEE diameter measurements and perforation avoided by careful en-bloc sharp resection from the base of the aortic valve to the papillar muscles. The incorporation of an oversized bovine pericardial patch into the anterior MV-leaflet ensures that the coaptation line is pushed posteriorly, which decreases the risk of residual valvular SAM. TEE should confirm an adequate and uncomplicated LVSM and AVV procedure.
Conclusions
This small series of a very limited patient population reflects the outcomes of the current surgical team of a single centre with extensive EPAS experience. Simultaneous LVSM and AVV surgery by EPAS is safe, effective and allow for durable LVOT gradient reduction, SAM- and AVV-surgery outcomes. Our series achieved a 100% long-term freedom from re-intervention and improved quality of life in all patients with no significant new or recurrent echocardiographic AVV pathology. EPAS offers the potential benefits associated with minimally invasive cardiac surgery and is an attractive alternative to conventional approaches for HOCM and concomitant AVV disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. F Van Praet and Dr. F Casselman serve on the Edwards Lifesciences Medical Advisory Board for minimally invasive cardiac surgery. Dr. J van der Merwe has no conflicts of interest to declare. This study was presented at EACTS, Vienna, 2017.
References
- Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733-79. [Crossref] [PubMed]
- Morrow AG, Reitz BA, Epstein SE, et al. Operative treatment in hypertrophic subaortic stenosis. Techniques and the results of pre and postoperative assessments in 83 patients. Circulation 1975;52:88-102. [Crossref] [PubMed]
- Heric B, Lytle BW, Miller DP, et al. Surgical management of hypertrophic obstructive cardiomyopathy. Early and late results. J Thorac Cardiovasc Surg 1995;110:195-206. [Crossref] [PubMed]
- Robbins RC, Stinson EB. Long-term results of left ventricular myotomy and myectomy for obstructive hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 1996;111:586-94. [Crossref] [PubMed]
- Ommen SR, Maron BJ, Olivotto I, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;46:470-6. [Crossref] [PubMed]
- Smedira NG, Lytle BW, Lever HM, et al. Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg 2008;85:127-33. [Crossref] [PubMed]
- Desai MY, Bhonsale A, Smedira NG, et al. Predictors of long-term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation 2013;128:209-16. [Crossref] [PubMed]
- Yu EH, Omran AS, Wigle ED, et al. Mitral regurgitation in hypertrophic obstructive cardiomyopathy: relationship to obstruction and relief with myectomy. J Am Coll Cardiol 2000;36:2219-25. [Crossref] [PubMed]
- Casselman F, Vanermen H. Idiopathic hypertrophic subaortic stenosis can be treated endoscopically. J Thorac Cardiovasc Surg 2002;124:1248-9. [Crossref] [PubMed]
- Chitwood WR. Idiopathic Hypertrophic Subaortic Septal Obstruction: Robotic transatrial and transmitral ventricular septal resection. Ann Cardiothorac Surg 2017;6:54-9. [PubMed]
- Sorajja P, Pedersen WA, Bae R, et al. First Experience with Percutaneous Mitral Valve Plication as Primary Therapy for Symptomatic Obstructive Hypertrophic Cardiomyopathy. J Am Coll Cardiol 2016;67:2811-8. [Crossref] [PubMed]
- Coylewright M, O'Neill ES, Robb JF, et al. Reduction of left ventricular outflow tract obstruction with transcatheter mitral valve repair. Echocardiography 2017;34:625-6. [Crossref] [PubMed]
- Casselman FP, Van Slycke S, Dom H, et al. Endoscopic mitral valve repair: feasible, reproducible, and durable. J Thorac Cardiovasc Surg 2003;125:273-82. [Crossref] [PubMed]
- van der Merwe J, Casselman F, Stockman B, et al. Endoscopic atrioventricular valve surgery in extreme obesity. Türk Göğüs Kalp Damar Cerrahisi Dergisi 2017;25:654-8. [Crossref]
- van der Merwe J, Casselman F, Stockman B, et al. Endoscopic atrioventricular valve surgery in adults with difficult-to-access uncorrected congenital chest wall deformities. Interact Cardiovasc Thorac Surg 2016;23:851-5. [Crossref] [PubMed]
- Casselman FP, LaMeir M, Jeanmart H, et al. Endoscopic mitral and tricuspid valve surgery after previous cardiac surgery. Circulation 2007;116:I270-5. [Crossref] [PubMed]
- van der Merwe J, Casselman F, Stockman B, et al. Late redo-port access surgery after port access surgery. Interact Cardiovasc Thorac Surg 2016;22:13-8. [Crossref] [PubMed]
- Casselman FP, Van Slycke S, Wellens F, et al. From classical sternotomy to truly endoscopic mitral valve surgery: a step by step procedure. Heart Lung Circ 2003;12:172-7. [Crossref] [PubMed]
- Coddens J, Deloof T, Hendrickx J, et al. Transesophageal echocardiography for port-access surgery. J Cardiothorac Vasc Anesth 1999;13:614-22. [Crossref] [PubMed]
- Gooris T, Van Vaerenbergh G, Coddens J, et al. Perfusion techniques for port-access surgery. Perfusion 1998;13:243-7. [Crossref] [PubMed]
Cite this article as: van der Merwe J, Casselman F, Van Praet F. Endoscopic Port AccessTM left ventricle outflow tract resection and atrioventricular valve surgery. J Vis Surg 2018;4:100.


