
Open surgical repair for the removal of an Amplatzer™ device
Introduction
Transcatheter closure of atrial septal defects (ASDs) using the Amplatzer™ Septal Occluder device (St. Jude Medical, Plymouth, MN) has been demonstrated to be safe and effective since the device was approved by the U.S. Food and Drug Administration (FDA) in 2001. However, several complications have been associated with the use of this device, including perforation, device migration, embolization, thrombosis, infection of the atrial wall, and tissue erosion. A case of cardiac erosion was published in 2002 (1). Erosion rates have since been reported to range from 0.043% to 0.3% (2).
The FDA issued a warning in 2013 about safety issues encountered with the Amplatzer™ device after receiving more than 100 reports of device-associated erosion (3). The device is made of a self-expanding nitinol metal mesh. If the device is sized improperly, the mesh can rub against the cardiac wall and erode the tissue of the atrium and the adjacent aortic root, potentially leading to cardiac tamponade.
Herein, we report the case of a patient who required open surgical repair for the removal of an Amplatzer™ Septal Occluder device that was eroding into the aortic root.
Case presentation
The patient was a 33-year-old woman who had undergone ASD repair with an Amplatzer™ device 9 years prior at age 24.
Patient selection and workup
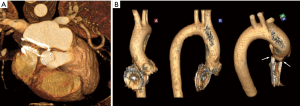
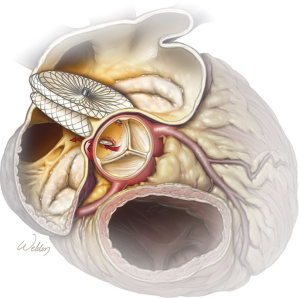
A preoperative transesophageal echocardiogram showed erosion consistent with a fistula from the noncoronary sinus of the aortic root to the right atrium. From preoperative computed tomography (CT) imaging, it was evident that the previously placed Amplatzer™ device had eroded into the noncoronary sinus of the aortic root at two distinct locations. This finding was confirmed with 3-dimensional CT reconstruction images (Figure 1). An illustration is provided to show the fistulas and the direction of blood flow through these defects (Figure 2).
Procedure
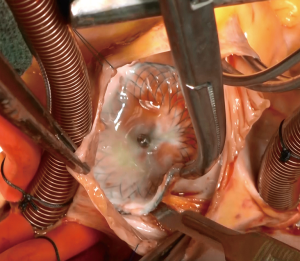
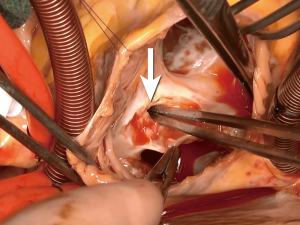
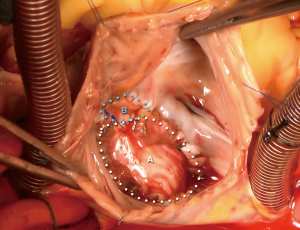
A median sternotomy was performed, and cardiopulmonary bypass was initiated using bicaval cannulation. A left ventricular sump was placed via the right superior pulmonary vein. The heart was arrested with antegrade and retrograde cardioplegia. A right atriotomy was made, and upon entry to the right atrium, the Amplatzer™ device was found to be adherent to both the atrial wall and the septum (Figure 3). Careful, sharp dissection with a scalpel was used to separate the device from the surrounding structures (Figure 4). During this dissection process, the fistula into the aortic root was identified (Figure 5). In order to remove the device from the surrounding structures, almost the entire atrial septum had to be removed. The fistula was debrided back to the healthy tissue and repaired with a piece of bovine pericardium, which was secured into place with a running 4-0 Prolene suture. After the first fistula was closed, the second opening into the aortic root was identified, and it was noted that there was damage to the noncoronary sinus that was not amenable to simple repair. The aortic root was opened and exposed via transection at the sinotubular junction. The aortic tissue of the noncoronary sinus was completely resected. The sinus was reconstructed using a piece of bovine pericardium. After the noncoronary sinus of the aortic root was repaired, the residual ASD was repaired using a piece of autologous pericardium. The residual defect of the superior portion of the right atrium was closed with a separate piece of bovine pericardium (Figure 6). The right atriotomy was then closed in a running fashion, and the aorta was reapproximated and closed with a running Prolene suture, which incorporated the free edge of the reconstructed noncoronary sinus. An intraoperative echocardiogram showed a completely competent aortic valve without residual fistualization.

Postoperative management
The patient recovered well and was discharged home on postoperative day 6 without any complications. An echocardiogram obtained 7 months after the repair showed no residual defects, and the patient was still doing well 16 months after the repair.
Discussion
The Amplatzer™ Septal Occluder is the most commonly used device for transcatheter closure of ASDs because it is simple to deploy, can be used for a large range of defect sizes, and has a high occlusion rate compared with other transcatheter devices (5). However, in 2012, the FDA Circulatory System Devices Panel convened an advisory committee to review the safety of ASD closure devices from all manufacturers because of accounts of such devices causing erosions (2). A multicenter cohort registry on congenital cases of ASD was used to compare the outcomes of 688 patients who underwent ASD closure with 1 of 3 devices (6). The study showed that cardiac erosion was an uncommon event (0.5% of patients, n=3) and occurred only after the use of the Amplatzer™ Septal Occluder device, which was used in most patients (n=566).
McElhinney et al. (2) compared the case details for all the patients reported to have developed an erosion after Amplatzer™ device implantation to those for patients who underwent ASD closure with the same device but did not develop an erosion. They found that the erosions were associated with deficient aortic or superior vena cava rims (less than 5 mm in any view), a larger absolute size of the Amplatzer™ Septal Occluder devices, a larger difference between the balloon-sized diameter and native ASD diameter, and a smaller patient weight:device size ratio. In a multivariable analysis, the factors found to be associated with cardiac erosion were deficiency of any rim, balloon-sized diameter more than 5 mm larger than the native ASD diameter, and patient weight:device ratio. Multiple stakeholder organizations, including the American Heart Association and the American College of Cardiology, have agreed that deficient aortic rim should be classified as a high-risk feature for ASD closures (2).
A multi-institutional study from the European Congenital Heart Surgeons Association reviewed the records of 56 patients who underwent surgery to address complications after transcatheter closure of an ASD (7). Of the 56 patients, 9 required surgical intervention to address aortic or atrial perforation or erosion. The most frequent indication for surgery was device embolization (n=29). The mortality rate associated with surgically addressing the device-related complications was significantly higher than that associated with primary surgical closure of the ASD. The use of transcatheter techniques has also been reported for the treatment of an aorta-to-right atrium fistula caused by Amplatzer™ device erosion of the tissue; the report describes a means to close the fistula without removing the device (8).
In conclusion, careful preoperative assessment and imaging are essential before proceeding with transcatheter insertion of an ASD closure device, and proper follow-up is critical for detecting potential complications, including erosions. As the above case illustrates, when erosion with fistula formation does occur, surgical removal of the Amplatzer™ Septal Occluder device and fistula repair can be performed with good outcomes.
Acknowledgments
The authors thank members of the Surgical Research Core of the Michael E. DeBakey Department of Surgery at Baylor College of Medicine: Susan Y. Green, MPH, and Hiruni S. Amarasekara, MS, for editorial support. At the Texas Heart Institute, we also thank the Section of Scientific Publications for providing editorial support and the Section of Visual Communication Services for videography.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Di Bartolomeo, Davide Pacini and Mohamad Bashir) for the series “Best Video Presentation Prize for the 9th Postgraduate Course - Surgery of the Thoracic Aorta” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.05.09). The series “Best Video Presentation Prize for the 9th Postgraduate Course - Surgery of the Thoracic Aorta” was commissioned by the editorial office without any funding or sponsorship. SAL reports grants, personal fees and other from Terumo Aortic, personal fees and other from Baxter Healthcare, personal fees and other from Biom'up and Acer Theraputics, personal fees and other from Medtronic, Inc. W.L. Gore & Associates, and Cytodorbants, Inc., outside the submitted work; and SAL serves as a consultant for Terumo Aortic and Baxter Healthcare; has served as an Advisory Board Member for Biom’up and Acer Therapeutics; serves as a principal investigator for clinical studies sponsored by Terumo Aortic, CytoSorbants, and Baxter Healthcare; and has served as a co-investigator for clinical studies sponsored by Medtronic, Inc., W.L. Gore & Associates, and Cytosorbants, Inc.. OP reports other from W.L Gore and associates, other from Terumo Aortic, outside the submitted work. JSC reports personal fees and other from Abbott Laboratories, grants, personal fees and other from Terumo Aortic, personal fees and other from Medtronic; WL Gore, other from Edwards Lifesciences; CytoSorbents; Baxter Healthcare;, outside the submitted work and JSC receives royalties from Terumo Aortic for the branched thoracoabdominal aortic aneurysm graft, which is unrelated to the present work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aggoun Y, Gallet B, Acar P, et al. Perforation of the aorta after percutaneous closure of an atrial septal defect with an Amplatz prosthesis, presenting with acute severe hemolysis. Arch Mal Coeur Vaiss 2002;95:479-82. [PubMed]
- McElhinney DB, Quartermain MD, Kenny D, et al. Relative risk factors for cardiac erosion following transcatheter closure of atrial septal defects: a case-control study. Circulation 2016;133:1738-46. [Crossref] [PubMed]
- U.S. Food and Drug Administration. Rare serious erosion events associated with St. Jude Amplatzer atrial septal occluder (ASO): FDA safety communication. Available online: http://wayback.archive-it.org/7993/20170722215736/https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm371145.htm
- Orozco-Sevilla V, Weldon SA, LeMaire SA, et al. Case of a 33-year-old woman who required open surgical repair for the removal of an AmplatzerTM Septal Occluder device because fistulas had formed through the right atrium and noncoronary sinus within her native aortic root. Asvide 2018;5:533. Available online: http://www.asvide.com/article/view/25149
- Astarcioglu MA, Kalcik M, Sen T, et al. Ceraflex versus Amplatzer occluder for secundum atrial septal defect closure. Multicenter clinical experience. Herz 2015;40:146-50. [Crossref] [PubMed]
- El-Said H, Hegde S, Foerster S, et al. Device therapy for atrial septal defects in a multicenter cohort: acute outcomes and adverse events. Catheter Cardiovasc Interv 2015;85:227-33. [Crossref] [PubMed]
- Sarris GE, Kirvassilis G, Zavaropoulos P, et al. Surgery for complications of trans-catheter closure of atrial septal defects: a multi-institutional study from the European Congenital Heart Surgeons Association. Eur J Cardiothorac Surg 2010;37:1285-90. [Crossref] [PubMed]
- Wan JY, Zhang GJ, Jiang SL, et al. Transcatheter closure of aorta-to-right atrium fistula caused by erosion of Amplatzer Septal Occluder. JACC Cardiovasc Interv 2017;10:e33-e35. [Crossref] [PubMed]
Cite this article as: Orozco-Sevilla V, Weldon SA, LeMaire SA, Preventza O, de la Cruz KI, Coselli JS. Open surgical repair for the removal of an Amplatzer™ device. J Vis Surg 2018;4:118.


