Carinal resection: technical tips
Introduction
Carinal resection (CR) is defined as the resection of the trachea-bronchial bifurcation with or without lung resection. CR and reconstruction without lung resection is very rare and it is usually indicated in case of primary tumors of the carina or the distal trachea. More frequently, CR is associated to lung resection for a non-small cell lung cancer (NSCLC) infiltrating the trachea-bronchial bifurcation; in this case, right tracheal sleeve pneumonectomy is usually the most frequent procedure, followed by left tracheal sleeve pneumonectomy or right upper lobectomy/bilobectomy with CR.
Despite the improvement of surgical and anesthetic techniques, CR is still considered one of the most challenging surgical resection and reconstruction. Reconstruction of the airway could be performed in different ways according to the extension of the resection and to the surgeon experience with the sole purpose to obtain a tension-free anastomosis to reduce as much as possible the possible post-operative complications due to impairment healing of the suture.
After the initial attempts of CR carried out in the 40s to demonstrate the feasibility of the technique, the first to show a clinical series of CR was Abbott in 1950 reporting four cases of right tracheal sleeve pneumonectomy (1). Since then, different authors described various techniques to re-anastomosis the airway after CR. In 1954, Crafoord (2) was the first to describe an anastomosis between the bronchus intermedius and the trachea after an upper lobectomy. In 1957, Barclay et al. (3) described an end-to-end anastomosed between the trachea and the right main bronchus and the left main bronchus was then anastomosed end-to-side to the bronchus intermedius; in 1963 Grillo (4) performed the same CR and reconstruction but with the end-to-side anastomosis of the left main bronchus to the trachea. Another technique was described by Eschapasse in 1967 (5) who performed an end-to-end anastomosis between distal trachea and left main bronchus, and an end-to-side anastomosis between the intermediate bronchus and the left main bronchus.
Patient selection and workup
CRs are usually performed in case of NSCLC, primitive airway tumors and benign lesions invading the carina.
Preoperative evaluation for all patients included clinical history, physical examination, routine blood tests, pulmonary and cardiac function test such as spirometry, arterial blood gas analysis, perfusion lung scan and electrocardiography.
Total body computed tomography (CT) and positron emission tomography with fluorodeoxyglucose (PET) are routinely performed to evaluate the lesion and the infiltration of the surrounding structures as well as to ensure the absence of a metastatic disease in case of malignancy.
Bronchoscopy is the most important pre-operative tool to identify the lesion and to plan the surgical resection. In fact, the diagnosis of the lesion is usually obtained by endobronchial biopsy or endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) which also allows to evaluating the extension of the tumor, to identify suspicious paratracheal tumor spreading and to assess mediastinal nodal involvement in case of suspicious of N2 disease on CT or PET scan. In case of histologically proven N2 disease, the patients should be candidates to induction chemotherapy and then re-staged with a total body CT and PET scan to schedule the responders or the patients with stable disease for surgery. Endobronchial biopsies are important in order to map the trachea and to detect the limits of surgical resection avoiding intraoperative surprises and tensions on the anastomosis. A tension free anastomosis is possible when the tumor does not extend beyond 2 cm of the lower trachea or more than 1.5 cm of the opposite main bronchus (6) or the distance between the lower trachea and the opposite bronchus is not more than 4 cm (7).
Cervical mediastinoscopy should be performed in order to confirm a suspected infiltration of the trachea-bronchial angle and to better plan the extension of the resection.
During a multidisciplinary meeting thoracic surgeons and oncologists confirmed patients’ resectability and the induction treatment plans, if needed.
In case of NSCLC with carinal involvement, the induction chemotherapy is indicated with the aim to downstage the tumor increasing the resectability and reducing the extension of the resection other than improving long term survival by controlling potential systemic micrometastasis. Furthermore, the patient has usually a better compliance to induction therapy rather than adjuvant therapy; patient’s performance status and clinical condition are usually better than after an extended surgery when post-operative complications could delay the beginning of the treatments. On the other hand, theoretical disadvantages of induction chemotherapy are a possible progression of the disease during the treatment making the patients inoperable, more surgical difficulties and increase of post-operative morbidity and mortality. In our experience, an absolute indication to induction chemotherapy in the case of carinal involvement is the presence of superior vena cava involvement as well as N2 disease.
Contraindication for CR are impairment respiratory or cardiac function, extension of the tumor that hamper a tension free anastomosis, multiple N2 or N3 lymph node stations involvement and presence of distant metastasis. Preoperative irradiation more than 45 Gy is not an absolute contraindication to CR by it should be avoided.
Pre-operative preparation
Before and during surgery a strict cooperation between surgeon and anesthesiologist is essential. An adequate anesthesia is in fact crucial to maintain the adequate gas exchange during surgical airway reconstruction. During tracheal surgery and/or carinal reconstruction a cross-field ventilation is necessary to maintain the general anesthesia, and it is usually alternated with intermittent apnea to facilitate the first part of the airway anastomosis (posterior wall); then, the cross-field ventilation is replaced with original endotracheal tube, which is pulled down into the bronchus beyond the anastomosis, allowing to complete the last part of the trachea-bronchial suture (anterior wall).
High frequency jet ventilation (HFJV) has been introduced as an alternative ventilation option thanks to the smaller endotracheal tube which allows an easier surgical reconstruction. In fact, HFJV is frequently performed via the lumen of the blocker tube which could be alternated in the main bronchus during each anastomosis. Thus, HFJV could be preferred to the cross-field ventilation above all in case of minimally invasive approach to the carina where the surgeon usually has a small operative area and a short anastomotic time (8). Besides, HFJV is also useful to maintain an adequate oxygenation to the contralateral lung during the cross-field ventilation.
Extracorporeal membrane oxygenation (ECMO) has also been reported with good results (9,10) in case of impossibility of endotracheal intubation such as severe tracheal stenosis or complete obstruction. A single lumen veno-venous ECMO could nowadays considered the first choice for airway surgery facilitating the surgery thanks to tubeless operation field and leading to a more accurate resection and reconstruction as well as a more definitive airway security.
Equipment preference card
- 3/0 Non-absorbable monofilament for the running suture of the anastomosis;
- heavy stiches between chin and chest to avoid tension on the anastomosis;
- anesthetic instrument for the cross-field ventilation or HFJV or ECMO;
- standard surgical instrument to perform tracheal and lung resection.
Procedure
The surgical approach to the carina could be performed differently, according to the position of the tumor and to the surgeon’s experience.
Right-sided lesions requiring a CR with pulmonary resection are better approached through an ipsilateral thoracotomy usually in IV intercostal space. Tumor involving the carina as well as the left main bronchus and requiring a left carinal pneumonectomy could be treated using a left thoracotomy with sub aortic dissection but only in case of very limited tracheal resection due to a bad exposure of the trachea after moving the aortic arch.
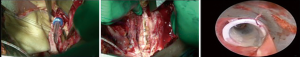
CR without pulmonary resection and left carinal pneumonectomy are better approached through a median sternotomy. In early 60’s, Padhi (11) and Abruzzini (12) were the first authors to describe the trans mediastinal approach to the trachea for the treatment of post-pneumonectomy fistula: the sternum was fully divided and the anterior pericardium opened vertically between the superior vena cava and the aorta; then, after opening the posterior pericardium, the retraction of the superior vena cava and the aorta allowed to expose a quadrilateral space in which the lower trachea and the carina could be easily approached (Figure 1).
Recently, minimally invasive thoracic surgery such as video-assisted thoracoscopic surgery (VATS) or robotic-assisted surgery (RATS) has been proposed for tracheal or CR and reconstruction. So far, only few cases (13-19) have been published in literature showing the feasibility of these minimally invasive techniques for tracheal and carinal surgery but still with several limitations mostly due to challenging learning curve, impossibility of palpation and difficult airway management.
CR with pulmonary resection
This type of surgery is uncommon and it is usually performed in case of NSCLC involving the origin of the main bronchus (right or left) and/or the carina. Considering the rarity of the tracheal sleeve pneumonectomy only few specialized center are able to develop the skill to perform this surgery. Besides, the infrequency of this surgery has also determined a lack of familiarity with indications and results so often these tumors are considered “unresectable” and treated only by chemo or chemo-radiotherapy.
A multidisciplinary team should always be involved to decide the right timing the surgery and the possible induction or adjuvant treatments to improve the long-term survival. The 5-year overall survival after CR ranges from 26% to 44% in different series, with an improved survival up to 50% in pN0 patients (20-26) showing the importance of the accurate selection of the patient. Another important positive prognostic factor is the completeness of the resection (21). Thus, induction chemotherapy should be proposed to reduce the rate of explorative thoracotomy, to improve R0 resection and lymph nodes down-staging as well as to reduce the incidence of recurrences and distant metastasis. Besides, induction therapy is better tolerated than adjuvant therapy in particular in this challenging surgery often related with a severe post-operative morbidity. In our experience, N2 disease should be considered an absolute indication to induction chemotherapy in the case of carinal involvement.
Cervical mediastinoscopy should be performed prior to surgery to evaluate the tracheobronchial angle and the patient’s resectability (27). Identification and isolation of the airway should always be performed first, before the vessels resection, to ensure the feasibility of the surgical resection.
Right tracheal sleeve pneumonectomy
The azygos vein is divided and the tracheobronchial bifurcation exposed. The distal trachea is dissected respecting the lateral blood supply and the left main bronchus is then divided first, followed by the transection of the distal trachea, to allow a cross-field ventilation of the left lung (27). This dissection must be done carefully to avoid injury of the left recurrent laryngeal nerve which lies below the aortic arch (27). Then, the end-to-end anastomosis between the distal trachea and the left main bronchus is performed maintaining the cross-field ventilation until the posterior part of the anastomosis is completed. The procedure is not indicated when more than 4 cm of the trachea have to be resected.
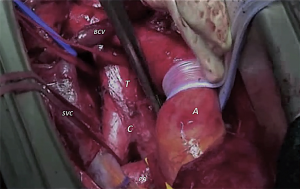
Considering the anatomical position of the distal trachea and the carina, the superior vena cava is also often involved by the tumor, and a contextually vascular resection and reconstruction is not infrequently associated with the CR (28) (Figure 2). In our recent study published by Galetta et al. (29), 15 patients out of 32 undergoing right tracheal sleeve pneumonectomy had a combined superior vena cava resection and reconstruction. In this study, we showed 5-year survival and disease free survival of 30.3% and 27.7%, respectively, significantly related to nodal down staging and adjuvant treatment (P=0.035 and P=0.007, respectively), whereas induction therapy was not associated to morbidity and mortality.

Left tracheal sleeve pneumonectomy
This procedure can be performed through a lateral thoracotomy only if no more than 1 cm of trachea and right main bronchus is involved below the margins of the left main bronchus or in case of unplanned surgery due to positive resection margin of the bronchus (27,29-31). On the left side, the dissection of the distal part of the trachea has to be performed beneath the aortic arch. Thus, to improve the exposure of the airway, the ligamentous arteriosus is divided and the aortic arch is dissected circumferentially, with particular attention to preserve the left laryngeal recurrent nerve. After identifying the trachea, the pretracheal plane is dissected and isolated followed by the right main bronchus (27). Traction sutures are usually placed in the left lateral wall of the trachea and in the median wall of the right main bronchus to facilitate the resection and the reconstruction. The right main bronchus is transected and a cross-field intubation is performed through the right main bronchus followed by the tracheal transection. Finally, anastomotic sutures are placed with an intermittent retraction of the aortic arch with spatula or umbilical tape around the vessel.
Instead, when a greater amount of the distal trachea is involved, the left carinal pneumonectomy is usually performed through a median sternotomy with a transpericardial approach which allows a better exposure of the surgical field with the mobilization of the ascending aorta up to the aortic arch and the transection of the ductus arteriosus.
Left carinal pneumonectomy is rarely performed, mostly due to the anatomic position of the left bronchus beneath the aortic arch and the frequent invasion of these structures from the tumor affecting the patient’s operability.
CR with right upper lobectomy or bilobectomy
The major problem with this resection is that the distance between the distal trachea and the left main bronchus after the excision should not be more than 4 cm, to avoid an excessive tension on the anastomosis (27). After the vessels for the right upper lobectomy are divided, the distal trachea and carina are isolated and divided as for a right carinal pneumonectomy (32); after the anastomosis between the trachea and the left main bronchus is completed, the bronchus intermedius, divided immediately below the origin of the right upper bronchus, is then anastomosed 1 cm below the initial anastomosis to the left main bronchus (Figure 3A). Only occasionally, the bronchus intermedius can be sutured to the lateral wall of the trachea if the length of the bronchus allows a tension-free anastomosis (Figure 3B) (32).
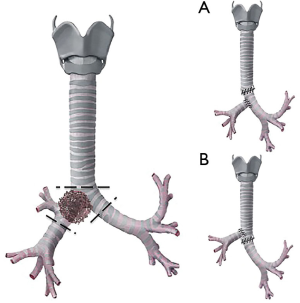
This procedure is indicated when a tumor of the right upper lobe involves the carina and the distal trachea without necessity to perform a right pneumonectomy.
CR without pulmonary resection
It is the ideal surgical resection in case of primary tumor of the airway involving only the carina or extended to a small part of the distal trachea.
Neo-carina
This CR is performed with the anastomosis of the median walls of the main bronchi to the end of the trachea, creating a new carina (27,32,33) (Figure 4). This type of reconstruction is the most similar to the physiological anatomy of the carina but the only limitation is the very limited mobility of neo-carina due to the anatomic relationship between the left main bronchus and the aortic arch which held down the bifurcation preventing major resection of the trachea (27,32).
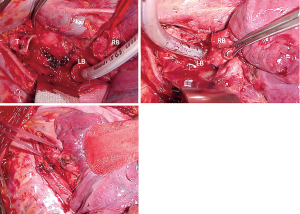
Thus, this reconstruction is indicated only in case of small tumors or benign lesions, confined only to the carina.
Barclay technique
This CR is performed with an end-to-end anastomosis between the trachea and the right main bronchus and then the creation of an ovoid opening into the medial wall of the bronchus intermedius to perform an end-to-side anastomosis of the left main bronchus with the bronchus intermedius (Figure 5). This reconstruction can be done only if the right main bronchus is sufficiently long to allow an adequate cross-field ventilation during the end-to-side anastomosis, otherwise it ends into a hypoventilation of the right lung (3).
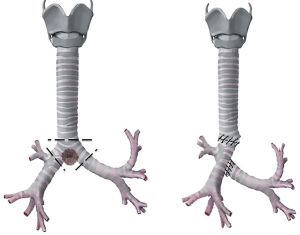
This type of reconstruction is usually indicated in case of large tumors that require a major resection of distal trachea.
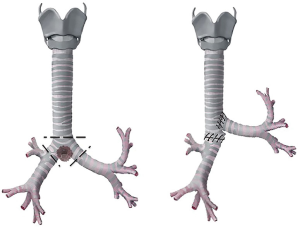
Grillo technique
This CR is similar both for indication and technique to the Barclay technique. The only difference is that the end-to-side anastomosis is performed between the left main bronchus and the lateral wall of the trachea making this procedure technically more challenging and only rarely indicated (4) (Figure 6).
Eschapasse or reverse Barclay technique
This CR is performed with an end-to-end anastomosis between the distal trachea and the left main bronchus; then, an opening on the right side of the tracheal wall, 1 cm above to the previous anastomosis, is performed and an end-to-side anastomosis between the trachea and the right main bronchus is completed (34) (Figure 7). Rarely, the second anastomosis could be performed between the right main bronchus and the medial wall of the left main bronchus, depending on length of the remaining right bronchus (5) (Figure 8).

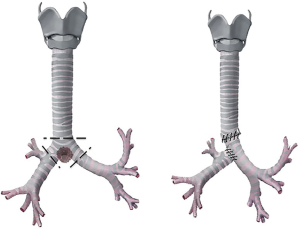
This CR is indicated when the tumor involves the carina and longer part of the trachea.
Our experience
In our experience we performed 45 CR: 32 right tracheal sleeve pneumonectomies, 4 right upper lobectomies with CR, 8 CR with neo-carina, and 1 CR according to reverse Barclay technique (Eschapasse). Thirty nine patients had NSCLC, 2 patients had a carcinoid tumor and 4 cases an adenoid cystic carcinoma. Major post-operative complications (one or more) occurred in 15 (33%) patients: 5 bronchopleural fistula, 4 acute respiratory distress syndrome (ARDS), 3 pulmonary edema, 2 hemothorax, 2 cardiac dislocation, 1 pneumonia, and 1 distal tracheal disruption. Minor post-operative complication occurred in 10 patients (22%) and the most frequent one was atrial fibrillation followed by anemia.
The overall 5-year survival rate patients was 27%, and up to 32% in down-staged NSCLC patients showing the importance of the induction therapy.
Role of team members
A well-organized team is essential to manage patients undergoing CR and it must involve several specialists experienced in diagnosis, operative treatment and postoperative care:
- Interventional pulmonologist performs bronchoscopy for the preoperative diagnosis and evaluation of tracheal infiltration as well as the post-operative follow up of the patients;
- Thoracic surgeon selects the patients and performs a technically challenging operation;
- Anesthesiologist manages the general anesthesia and endotracheal tube during surgery;
- Physiotherapist takes care of patients after the surgery to avoid the bronchial secretions retention and to improve the respiratory function;
- Oncologist and radiotherapist in collaboration with the surgeon determines the proper patient’s therapeutic pathway before and after surgery.
Post-operative management
It is desirable that the patient is extubated immediately after the operation. The positive pressure ventilation can apply stress on the airway anastomosis, increasing the post-operative morbidity (35). Heavy stitches between the chin and the upper part of the chest are recommended to maintain a mild degree of cervical flexion and to avoid uncontrolled head and neck movements which could over stretch the anastomosis in the early post-operative period. The chest tube and the stitches are usually removed on post-operative day 7th, after a bronchoscopic checked of the anastomosis. The first follow-up of the patient could be scheduled after 1 month with chest X-Ray and bronchoscopy.
Based on tumor histology and the pathological staging the patient is referred to the oncologist and/or radiotherapist for the further medical treatments.
Tips, tricks and pitfalls
- Different techniques have already been proposed to perform the tracheo-bronchial anastomosis. Whereas some authors prefer interrupted suture (35-37), others rather to reconstruct the airway continuity with a running suture of the membranous part associated to an interrupted suture of the cartilaginous part (38). Also the materials have been described differently by the various authors ranging from absorbable material to prevent granuloma formation or stenosis of the anastomosis (usually for the cartilaginous part) to non-absorbable stiches (for the membranous part). We usually performed the end-to-end anastomosis of the airway using two running sutures with a 3/0 non-absorbable monofilament: the stitches were positioned at the far side of the cartilaginous wall and subsequently fixed; afterwards, the two running sutures, first passed and secondarily tied, were performed obtaining the anastomosis;
- Anastomotic tension is the biggest cause of post-operative anastomotic complications. However, regardless of the different reconstruction techniques, any lung sparing CR determines a shortening of the longitudinal axis of the trachea-bronchial tree with a consequent tension on the anastomosis.
- If the resection is less than 2 cm, the longitudinal elasticity of the trachea, associated with the flexion of the neck, usually allows a tension-free carinal reconstruction; Besides, a release of the pretracheal plane could provide more mobility of the trachea, always respecting the lateral blood supply;
- If the resection is more than 2 cm, it is important to associate the CR with major release maneuvers to reduce the tension on the tracheobronchial anastomosis (33). On both sides, the section of the pulmonary ligament and the mobilization of the hilum, in particular the inferior vein which is physiologically short, can significant reduce the anastomotic tension (33); then, a U-shaped incision performed in the pericardium beneath the hilum, with intrapericardial division of the raphe, and eventually extended around the hilar vessels could increase further the hilum release (39).
- Reinforcing single stitches could be placed laterally to reduce the tension on the anastomosis;
- Tracheobronchial blood supply should be preserved as much as possible during dissection and release maneuvers to facilitate an adequate healing of the airway.
- The anastomosis should always be cover with a vascularized flap (muscular, pleural or pericardial fat tissue) (35) in case of induction therapy, especially radiotherapy, which might reduce the vascularization of the airway with a consequent tissue ischemia and increased risk of fistula;
- Chronic use of steroids might lead to an impaired healing of anastomosis, so the patient should be weaned from the steroids at least 2 to 4 weeks before surgery (35);
- The patients should be extubated as soon as possible after surgery considering that mechanical ventilation after surgery might increase the post-operative morbidity due to the positive pressure on the airway anastomosis (35);
- The morbidity rate after CRs usually ranges between 11% and 50% (20-23,26,40-42). The major causes of post-operative complications are usually related to an impaired healing of anastomosis and could be divided into two major categories:
- Fistula which could be bronchopleural or bronchovascular.
- Bronchopleural fistula is classified according to the time of onset after the operation in early (1–7 days), intermediate (8–30 days) and late (more than 30 days) (43). Early and intermediate bronchopleural fistula is usually repaired with direct suture of the anastomotic defect either through the same thoracotomy or through a transmediastinal “Abruzzini” approach. In case of CR with pulmonary resection, the bronchial stump has to be identified and the previous suture removed. Then, the new bronchial stump is sutured using non-absorbable 3-0 monofilament running in a U fashion and reinforced with 2 separate absorbable bands 7.5 mm in width (PDS-band) to avoid bronchial-wall laceration (44). Fibrin glue and vascularized muscle or pericardial fat flap could be used to cover and protect the new bronchial suture. In case of late fistula, according to the size (<8 mm) or the general post-operative condition of the patient, a bronchoscopic approach using of fibrin glue, bronchial stent or silver nitrate should be attempted. However, in case of conservative treatments failure or larger fistulas, open-window thoracostomy is often the only possible solution to guarantee the closure of the airway continuity and to resolve the infection of the pleural cavity, despite its major aesthetic and functional impact on the patient. In our experience, we also tried in an hopeless case of total disruption of the distal trachea after right carinal pneumonectomy, notwithstanding several failed attempts of reconstruction, a salvage surgery with reconstruction of the airway with free skin patch cover by vascularized omental flap and endobronchial silicon prosthesis (Figure 9).
- Recently, Petrella et al. reported successful BPF closure by autologous mesenchymal stromal cells endoscopic transplantation in a large animal model (45) as well as in clinical setting after extrapleural pneumonectomy for mesothelioma (46).
- Bronchovascular fistula is usually a catastrophic event and it is rarely identified before it is too late to be repaired;
- Stenosis which could be benign due to granulomas or inflammatory stricture or neoplastic due to tumor recurrence on the anastomotic margins. These are usually treated with conservative endoscopic dilatation or endoprothesis.
- Fistula which could be bronchopleural or bronchovascular.
- The CRs mortality ranges between 3% and 20% and it is related to pneumonia and ARDS or anastomotic failure (20-23,26,40-42).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Federico Rea) for the series “Tracheal Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.05.23). The series “Tracheal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consents were obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- ABBOTT OA. Experiences with the surgical resection of the human carina, tracheal wall, and contralateral bronchial wall in cases of right total pneumonectomy. J Thorac Surg 1950;19:906-22. [PubMed]
- Crafoord C, Bjork VO, Hilty H. Bronchial resection and broncho-tracheal anastomosis in tuberculous bronchial stenosis; report of a case. Thoraxchirurgie 1954;2:1-7. [PubMed]
- BARCLAY RS. McSWAN N, WELSH TM. Tracheal reconstruction without the use of grafts. Thorax 1957;12:177-80. [Crossref] [PubMed]
- Grillo HC, Bendixen HH, Gephart T. Resection of the carina and lower trachea. Ann Surg 1963;158:889-93. [Crossref] [PubMed]
- Eschapasse H, Vahdat F, Gaillard J, et al. Reflections on resection of the lower trachea and bronchial bifurcation. Ann Chir Thorac Cardiovasc 1967;6:63-70. [PubMed]
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve pneumonectomy. Thorac Surg Clin 2004;14:183-90. [Crossref] [PubMed]
- Dartevelle P, Macchiarini P. Techniques of pneumonectomy. Sleeve pneumonectomy. Chest Surg Clin N Am 1999;9:407-17. xi. [PubMed]
- Ujiie H, Yasufuku K. New era of "resection of the carina and lower trachea". J Thorac Dis 2017;9:4932-6. [Crossref] [PubMed]
- Keeyapaj W, Alfirevic A. Carinal resection using an airway exchange catheter-assisted venovenous ECMO technique. Can J Anaesth 2012;59:1075-6. [Crossref] [PubMed]
- Lei J, Su K, Li XF, et al. ECMO-assisted carinal resection and reconstruction after left pneumonectomy. J Cardiothorac Surg 2010;5:89. [Crossref] [PubMed]
- Padhi RK, Lynn RB. The management of bronchopleural fistulas. J Thorac Cardiovasc Surg 1960;39:385-93. [PubMed]
- Abruzzini P. Trattamento chirurgico delle fistole del bronco principale consecutive a pneumonectomia per tubercolosi. Chir Toracica 1961;14:165-71.
- Li J, Wang W, Jiang L, et al. Video-Assisted Thoracic Surgery Resection and Reconstruction of Carina and Trachea for Malignant or Benign Disease in 12 Patients: Three Centers' Experience in China. Ann Thorac Surg 2016;102:295-303. [Crossref] [PubMed]
- Nakanishi R, Yamashita T, Muranaka K, et al. Thoracoscopic carinal resection and reconstruction in a patient with mucoepidermoid carcinoma. J Thorac Cardiovasc Surg 2013;145:1134-5. [Crossref] [PubMed]
- Jiao W, Zhu D, Cheng Z, et al. Thoracoscopic tracheal resection and reconstruction for adenoid cystic carcinoma. Ann Thorac Surg 2015;99:e15-7. [Crossref] [PubMed]
- Xu X, Chen H, Yin W, et al. Thoracoscopic half carina resection and bronchial sleeve resection for central lung cancer. Surg Innov 2014;21:481-6. [Crossref] [PubMed]
- Qiu T, Zhao Y, Song J, et al. Two-port approached thoracoscopic carina reconstruction using natural bronchial bifurcation. J Cardiothorac Surg 2016;11:147. [Crossref] [PubMed]
- He J, Wang W, Li J, et al. Video-assisted thoracoscopic surgery tracheal resection and carinal reconstruction for tracheal adenoid cystic carcinoma. J Thorac Dis 2016;8:198-203. [PubMed]
- Jiao W, Zhao Y, Luo Y, et al. Totally robotic-assisted non-circumferential tracheal resection and anastomosis for leiomyoma in an elderly female. J Thorac Dis 2015;7:1857-60. [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Clinical experience with carinal resection. J Thorac Cardiovasc Surg 1999;117:39-52; discussion 52-3. [Crossref] [PubMed]
- Regnard JF, Perrotin C, Giovannetti R, et al. Resection for tumors with carinal involvement: technical aspects, results, and prognostic factors. Ann Thorac Surg 2005;80:1841-6. [Crossref] [PubMed]
- Roviaro G, Vergani C, Maciocco M, et al. Tracheal sleeve pneumonectomy: long-term outcome. Lung Cancer 2006;52:105-10. [Crossref] [PubMed]
- de Perrot M, Fadel E, Mercier O, et al. Long-term results after carinal resection for carcinoma: does the benefit warrant the risk? J Thorac Cardiovasc Surg 2006;131:81-9. [Crossref] [PubMed]
- Macchiarini P, Altmayer M, Go T, et al. Technical innovations of carinal resection for nonsmall-cell lung cancer. Ann Thorac Surg 2006;82:1989-97; discussion 1997.
- Rea F, Marulli G, Schiavon M, et al. Tracheal sleeve pneumonectomy for non small cell lung cancer (NSCLC): short and long-term results in a single institution. Lung Cancer 2008;61:202-8. [Crossref] [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Resection for bronchogenic carcinoma involving the carina: long-term results and effect of nodal status on outcome. J Thorac Cardiovasc Surg 2001;121:465-71. [Crossref] [PubMed]
- Grillo HC. Surgery of the Trachea and Bronchi, 1st edn. Hamilton, London: BC Decker Inc., 2004.
- Casiraghi M, Mariolo AV, Galetta D, et al. Right tracheal sleeve pneumonectomy and superior vena cava resection and reconstruction with bovine pericardial patch. Asvide 2018;5:546. Available online: http://www.asvide.com/article/view/25263
- Galetta D, Spaggiari L. Early and Long-Term Results of Tracheal Sleeve Pneumonectomy for Lung Cancer After Induction Therapy. Ann Thorac Surg 2018;105:1017-23. [Crossref] [PubMed]
- Maeda M, Nakamoto K, Tsubota N, et al. Operative approaches for left-sided carinoplasty. Ann Thorac Surg 1993;56:441-5; discussion 445-6. [Crossref] [PubMed]
- Salzer GM, Müller LC, Kroesen G. Resection of the tracheal bifurcation through a left thoracotomy. Eur J Cardiothorac Surg 1987;1:125-8. [Crossref] [PubMed]
- de Perrot M, Fadel E, Dartevelle P. Carinal resection, In: Patterson GA, Cooper JD, Deslauriers J, et al., editors. Pearsons’s Thoracic and Esophageal Surgery, 3rd edition. Churchill, PA: Livingstone Elsevier, 2008:383-92.
- Chirurgie de La Trachee et des Bronches. Encyclopedie Medico-Chirurgicale. Cou Thorax. Vol.1, 2nd edition. Paris: Editions Techniques, 1990:1-5.
- Casiraghi M, Mariolo AV, Galetta D, et al. Carinal resection is performed with an end-to-end anastomosis between the distal trachea and the left main bronchus and an end-to-side anastomosis is performed between the trachea and the right main bronchus. Asvide 2018;5:547. Available online: http://www.asvide.com/article/view/25264
- Tapias LF, Ott HC, Mathisen DJ. Complications Following Carinal Resections and Sleeve Resections. Thorac Surg Clin 2015;25:435-47. [Crossref] [PubMed]
- Frist WH, Mathisen DJ, Hilgenberg AD, et al. Bronchial sleeve resection with and without pulmonary resection. J Thorac Cardiovasc Surg 1987;93:350-7. [PubMed]
- Hsieh CM, Tomita M, Ayabe H, et al. Influence of suture on bronchial anastomosis in growing puppies. J Thorac Cardiovasc Surg 1988;95:998-1002. [PubMed]
- Weder W, Inci I. Carinal resection and sleeve pneumonectomy. J Thorac Dis 2016;8:S882-8. [Crossref] [PubMed]
- Lanuti M, Mathisen DJ. Carinal resection. Thorac Surg Clin 2004;14:199-209. [Crossref] [PubMed]
- Tedder M, Anstadt MP, Tedder SD, et al. Current morbidity, mortality, and survival after bronchoplastic procedures for malignancy (Internet). Ann Thorac Surg 1992;54:387-91. [Crossref] [PubMed]
- Porhanov VA, Poliakov IS, Selvaschuk AP, et al. Indications and results of sleeve carinal resection. Eur J Cardiothorac Surg 2002;22:685-94. [Crossref] [PubMed]
- Eichhorn F, Storz K, Hoffmann H, et al. Sleeve pneumonectomy for central non-small cell lung cancer: indications, complications, and survival. Ann Thorac Surg 2013;96:253-8. [Crossref] [PubMed]
- Varoli F, Roviaro G, Grignani F, et al. Endoscopic treatment of bronchopleural fistulas. Ann Thorac Surg 1998;65:807-9. [Crossref] [PubMed]
- Mazzella A, Pardolesi A, Maisonneuve P, et al. Bronchopleural Fistula After Pneumonectomy: Risk Factors and Management, Focusing on Open-Window Thoracostomy. Semin Thorac Cardiovasc Surg 2018;30:104-13. [Crossref] [PubMed]
- Petrella F, Toffalorio F, Brizzola S, et al. Stem cell transplantation effectively occludes bronchopleural fistula in an animal model. Ann Thorac Surg 2014;97:480-3. [Crossref] [PubMed]
- Petrella F, Spaggiari L, Acocella F, et al. Airway fistula closure after stem-cell infusion. N Engl J Med 2015;372:96-7. [Crossref] [PubMed]
Cite this article as: Casiraghi M, Mariolo AV, Galetta D, Petrella F, Brambilla D, Spaggiari L. Carinal resection: technical tips. J Vis Surg 2018;4:122.