Thoracoabdominal aortic aneurysms open repair: a multimodal approach
Introduction
A thoraco-abdominal aortic aneurysm (TAAA) involves the aorta at the visceral level and variably extends proximally and/or distally from this point (1). TAAA open repair consists of graft replacement with reattachment of the aortic branches. A multimodal approach with different adjuncts has progressively evolved to maximize organ protection and reduce surgical trauma. Prognosis following TAAA open repair varies according to the type of aneurysm undergoing repair, with extent I, II and III carrying a higher intraoperative and postoperative complication rate, especially regarding to spinal cord (SC) ischemia, pulmonary complication and renal failure. Surgical indication must, therefore, take into account these severe and life-threatening complications, which must be balanced against the risk of aneurysm rupture. Careful patient assessment with evaluation of comorbidities, surgical techniques, and current guidelines for preoperative, intraoperative and postoperative management of these patients had in the last decades a positive impact on the morbidity and mortality rate associated with TAAA open repair, allowing for better outcome compared to the past.
Preoperative imaging
Modern imaging is fundamental for risks evaluation, treatment planning, and follow-up of TAAA. In the past, X-ray radiography, followed by invasive catheter aortography, was the standard imaging approach for this disease. Nowadays, with the availability of a higher number of modalities, is possible to choose among a spectrum of different technologies tailoring the most appropriate imaging test for each clinical situation. At present, preoperative diagnostic tools are ultrasonography [Doppler ultrasonography, trans-thoracic echocardiography (TTE), and trans-esophageal echocardiography (TEE)], magnetic resonance imaging (MRI), and computerized tomography (CT). CT is nowadays the most widely used technique due to its higher diagnostic accuracy and availability compared to the others.
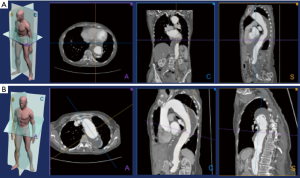
Imaging analysis is usually performed with the aid of a dedicated workstations that allow to analyze individual images with different post-processing techniques, such as, maximum intensity projection reformation (MIP), multiplanar volume reformation (MPR), volume rendering (VR), and 3D reformation (Figure 1).
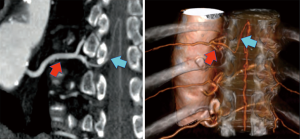
Perioperative SC ischemia is one of the possible most dramatic events after TAAA repair. An accurate knowledge of SC vascularization could be useful for risk stratification and procedure planning (Figure 2).
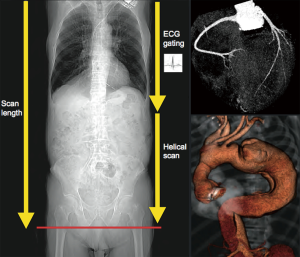
In the last decades, multi-detector computed tomography (MDCT) technology has developed significantly allowing non-invasive high-resolution imaging of the coronary circulation. At present, it is possible to study the coronary tree and the entire aorta with a small amount of contrast within a single breath-hold (2). For preoperative cardiac risk stratification during TAAA diagnostic workup, coronary-CT may become the standard imaging modality, with a faster and easier decision-making process (Figure 3).
Overall, nowadays CTA allows classification of TAAA and precise treatment planning.
Open surgery: tips & tricks
Preparation for surgery
TAAA open surgical repair requires strong cooperation between different specialists, in particular the surgeon, the cardiac anesthesiologist and the cardiovascular perfusionist. A right radial arterial catheter and a central venous access are placed before induction of general anesthesia to monitor and optimize cardiac function and patient’s hemodynamics. During the surgical procedure, TEE is routinely used to detect cardiac wall-motion alterations and to evaluate the patient’s volume status. Among two large-bore intravenous catheters inserted, one could be connected to a rapid infusion system. Autotransfusion devices (e.g., Cell Saver, Braintree, MA, USA) are needed during TAAA open surgery to reduce the need for banked blood (3).
SC drainage
After induction of general anesthesia, the patient is positioned in right lumbar flexion and a lumbar puncture is performed, with an aseptic fashion, for spinal drain insertion. An intervertebral space at the level of the iliac crest is normally used to avoid the lower limit of SC extension and prevent possible lesions. A 14 G Tuohy needle is used for lumbar puncture and a drainage catheter is placed into the subarachnoid space approximately 10 centimeters beyond the tip of the needle. The drain catheter is then secured to the patient (Figure 4) and connected to a dedicated system equipped with a roller pump that allow a volume-controlled cerebro-spinal fluid drainage (CSFD), the LiquoGuard automated device (Möller Medical GmbH, Fulda, Germany) that maximize the safety of drainage (4).
After inserting the catheter for CSFD, the patient is then positioned for the surgical procedure. With a right lateral decubitus, the shoulders are oriented at 60° and the hips at 30°. This position allows for access both left thorax, abdomen and both the groin. A moldable beanbag with suction and vacuum creation is used to maintain patient position during the procedure. In order to maintain a permissive hypothermia a circulating water mattress is placed between the beanbag and the patient.
A Robertshaw tube (double-lumen endo-bronchial tube) is used to exclude left lung ventilation in order to obtain an adequate thoracic aorta exposure. Fiberoptic bronchoscopy could be used to check the position of the endotracheal tube especially in patients with distortion of the trachea or the left main bronchus caused by large TAAA. Correct position should be re-detached after final positioning of the patient. A right femoral artery line is placed before surgery to monitor the arterial pressure during aortic clamping time and left heart bypass (LHBP).
Surgical technique
Thoraco-phreno-laparotomy
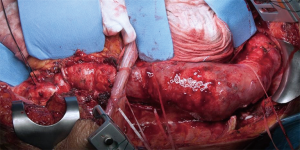
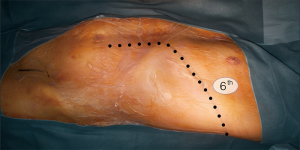
The thoracic incision is frequently performed in 5th, 6th or 7th intercostal space but could vary in level and length depending on the needing of aortic exposure and aneurysm extent (Figure 5). The incision crosses the costal margin with a gentle curve to reduce the risk of tissue necrosis (Figure 6).
A posterior section of the rib associated with a gently and progressive retraction are able to reduce thoracic trauma and fractures. When extensive exposure is needed a combined proximal and distal resection of the rib could be performed. When the left lung is excluded from ventilation the pleural space in entered and right mono-pulmonary ventilation is maintained during thoracic aorta replacement.
The radial division of the phrenic center may lead to left hemi-diaphragm paralysis which significantly increase the respiratory weaning time and the risk of postoperative respiratory failure. For this reason, a limited left circumferential section of the diaphragm in its muscular part is routinely carried out, sparing the phrenic center (Figure 7).
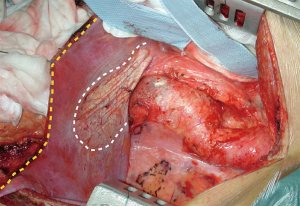
During proximal thoracic aortic neck isolation, the TEE probe or a large caliber oro-gastric tube can be helpful in order to identify the esophagus and avoid possible lesions. Then, the aorta is generally supported using a vessel-loop.
During distal aortic arch and proximal descending aorta isolation and clamping maneuvers, the vagus nerve and the origin of the recurrent laryngeal nerve are identified in order to preserve them. Intercostal arteries from proximal thoracic aorta are identified and clipped to facilitate the preparation for the proximal anastomosis. The visceral abdominal aorta is exposed through a transperitoneal approach; the left colon is mobilized with parieto-colic space incision and a complete medial visceral rotation is performed so that the left colon, the spleen and the left kidney can be retracted anteriorly and to the right. Transperitoneal approach allows direct view of the abdominal organs to evaluate the efficacy of revascularization at the end of aortic repair. Extra care must be taken to avoid damage to the spleen that is particularly prone to bleed even if only small capsular lesions are produced.
Distal aortic perfusion (DAP)
Aortic cross-clamping at thoracic level may lead to severe cardiac afterload and cause distal ischemia. To avoid these hemodynamic disturbances, techniques for distal aortic perfusion with LHBP are useful during TAAA open repair. The centrifugal pump (Biomedicus) of LHBP is able to provide flow to the SC, viscera and kidneys during the aortic cross-clamp period with concomitant reduction of proximal hypertension and cardiac afterload (Figure 8). A non-occlusive retrograde cannulation of the common femoral artery is used for distal perfusion (Figure 9). A low dose intravenous heparin is administered during LHPB and clamping time to reduce bleeding from the extensive tissue exposure.
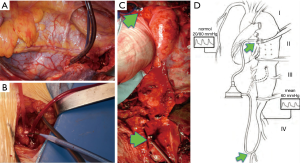
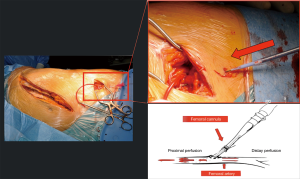
Spinal cord perfusion pressure optimization using techniques, such as proximal aortic pressure maintenance and distal aortic perfusion, is reasonable as an integral part of the surgical, anesthetic, and perfusion strategy in open and endovascular thoracic aortic repair patients at high risk of spinal cord ischemic injury. Institutional experience is an important factor in selecting these techniques. (Class IIa; level of evidence: B) (5).
The arterial blood could be drained from superior (or inferior) left pulmonary vein that is usually directly cannulated with a 22 Fr cannula. The oxygenated blood is then re-infused through a centrifugal pump into the left femoral artery with a 14/16 Fr non-occlusive percutaneous femoral cannula (6). A “Y” bifurcation with two occlusion/perfusion catheters (9 Fr) is connected to the circuit for visceral vessels selective perfusion (Figure 10).
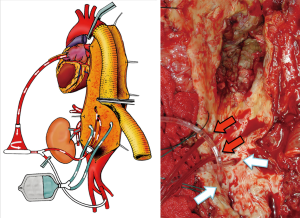
Aortic repair
After surgical preparation the TAAA is exposed (Figure 11). The proximal portion of the TAAA is cross-clamped proximally and distally and the aorta is circumferentially transected (Figure 12). Special care must be taken to avoid possible esophagus lesions during this maneuver.
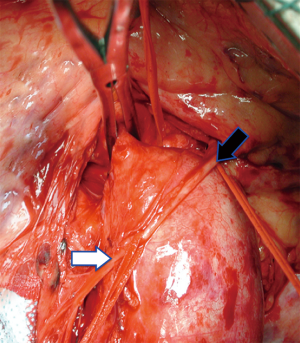
In case of degenerative TAAA a 2/0 monofilament polypropylene is routinely used to suture a dacron graft to the descending thoracic aorta in a running fashion. In case of fragile aorta or dissection a 3/0 monofilament polypropylene could be preferred. The suture is reinforced with Teflon pledgets or Teflon felt (Figure 13).
When the proximal anastomosis is complete the proximal clamp is removed, the LHBP is temporarily interrupted and the distal clamp is reapplied distally above the visceral vessels (sequential cross-clamping). Once the aortotomy is distally extended, critical patent segmental arteries from T7 to L2 could be identified and temporarily occluded with 4 Fr Pruitt catheters to avoid blood steal phenomenon (Figure 14). Feasible arteries are reattached to the graft by means of aortic patch or graft interposition. Intraoperative monitoring of SC motor evoked potentials (MEP) and somatosensory-evoked potential (SSEP) are established tools that can be used to guide intercostal arteries research and reattachment during TAAA repair procedures and may potentially minimize SC ischemia (7).
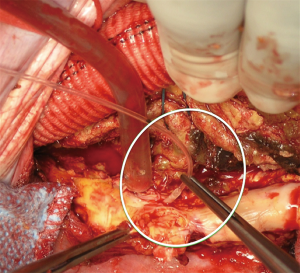
Moderate systemic hypothermia is reasonable for protection of the spinal cord during open repairs of the TAAA. (Class IIa; level of evidence: B) (5).
The distal clamp is moved distally at the infrarenal level and the aneurysm is opened below the diaphragm. At this moment the celiac trunk and the superior mesenteric artery are selectively cannulated with 9 Fr. irrigation-perfusion catheters (LeMaitre Vascular) and perfused with hematic perfusion through the pump (400 mL/min).
Previous studies have demonstrated the protective effects on renal function of hypothermia and of renal artery perfusion with cold crystalloid solutions (8). Both perfusion with isothermic and cold blood failed to support the hypothesis that blood perfusion is more effective in renal protection than cold crystalloid. In the search of the optimal solution for kidney protection we recently introduced cold perfusion of both renal arteries with Custodiol (Histidine-Tryptophan-Ketoglutarate) solution.
We reported a cohort of 104 consecutive patients treated for a thoracoabdominal aneurysm: 50 (48%) had renal perfusion with Custodiol and 54 (52%) with lactated Ringer’s solution. Freedom from acute kidney injury was significantly increased in the Custodiol group (38.1% vs. 9.5%; P=0.002) despite longer total renal ischemic time (51.5±16.4 vs. 43.6±16.0 minutes; P=0.05). A significant upward trend of perioperative estimated glomerular filtration rate was observed in the Custodiol group (group × time interaction = F3,66; P<0.001), and by multivariate analysis, Custodiol perfusion was the only independent predictor of non-AKI (P=0.04). The use of Custodiol in this study was safe and provided improved perioperative renal function compared with lactated Ringer’s solution. Randomized trials are needed to confirm these data and to assess their clinical consequences (9).
Preoperative hydration and intraoperative mannitol administration may be reasonable strategies for preservation of renal function in open repairs of the descending aorta. (Class IIb; level of evidence: C) (5).
Traditional methods for visceral arteries reimplantation include direct reattachment to a tailored side cut on the aortic graft (inclusion technique) (Figure 15). The inclusion of visceral and renal vessels in an aortic patch (VAP), compared with single vessel reattachment, decrease the number of anastomoses and the duration of organ ischemia.
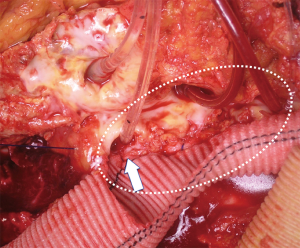
However, in case of considerable distances between the aortic branches ostia, or in case of connective tissue disorders, the aortic patch tissue may result in subsequent aneurysmal degeneration in time.
To reduce the VAP size, distant visceral vessels could be reattached separately directly or with graft interposition. The left renal artery normally arises distally from the others ostia and is frequently not included in the VAP with reduced risks of late aneurysmal degeneration. The disadvantage of visceral and renal arteries separate reattachment is that a higher number of anastomoses is required, and the procedure may be technically more demanding.
When separate vessels reattachment is needed, pre-shaped aortic multi-branched grafts reduce the number of total anastomoses to be performed (Figure 16). These grafts play a major role when repairing an aneurysm in presence of a connective disease (e.g., Marfan syndrome), however its use may be more tricky and time-consuming.
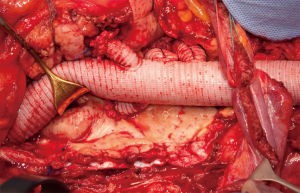
The atherosclerotic aortic degenerative disease in patients with TAAAs often involves also the aortic branches that arise at visceral level with stenosis or even occlusion. Visceral and renal vessels could also be involved by the lamella in case of dissecting TAAA. All these situations cause flow impairment, represent significant predictor of renal complications after TAAA repair, and need to be managed during open repair (10). During vessels reattachment, the presence of calcification and thrombus at the origin of the renal and visceral arteries may lead to plaque disruption and dissection with subsequent visceral and kidney malperfusion. Thus, in case of severe stenosis, an endarterectomy of the ostia is required before revascularization to improve patency rate. However, this maneuver may lead to severe complications such as vessel thrombosis, distal dissection, or even perforation of the friable endarterectomized.
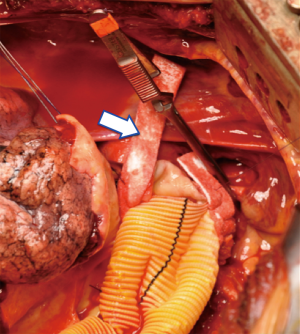
To overcome this problem, an alternative management of aortic branches stenosis is represented by direct stenting of the visceral and renal vessels during TAAA open repair. Under direct vision a bare metal stent is positioned and expanded within the artery addressing arterial stenosis, dissection and the possibility to tack down an unsatisfactory end point after visceral endarterectomy (Figure 17). Several groups reported their experience with the use of bare stents during TAAA open repair (11,12).
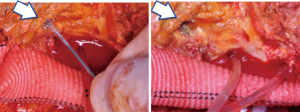
An alternative solution to these challenging anatomies may be the use of covered self-expanding stents for sutureless anastomoses. Initially described for visceral revascularization during hybrid surgery for complex aortic repair, we recently described the routine use of this technique to reattach the left renal artery with several technical advantages (13) (Figure 18). In our experience, short-term clinical and radiologic outcomes were satisfactory, however larger series and longer follow-up are needed to confirm the safety and durability of the proposed technique.
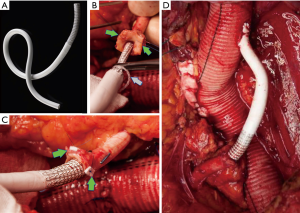
After restoring antegrade flow to the visceral and renal vessels, an end-to-end anastomosis with the distal aorta is performed and the last clamp removed (Figure 19).
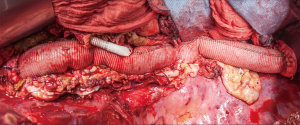
Postoperative management and results
Postoperative care
At the end of the surgical procedure, the patient is transferred to the intensive care unit (ICU) for postoperative management of blood pressure, heart rate, respiratory rate, and urine output. The CSFD is continued for 48–72 hours after the procedure to reduce SC edema and minimize delayed-onset paraplegia. Blood products are often necessary in the postoperative period to optimize the patient’s hemodynamics and correct any residual coagulopathy. Clinical management of acute moderate to severe bleeding is one of the major challenges for ICU team. Though substitution of erythrocytes by transfusion of red blood cells (RBC) is a routine task, adequate maintenance of haemostasis may be considerably more demanding. In fact, the underlying cause of bleeding and subsequent treatment may be completely different depending on the clinical scenario. Standard plasmatic coagulation tests such as PT/INR, aPTT and plasma fibrinogen level have several major limitations for their use in guiding perioperative management of bleeding disorders. Therapeutic options for effective haemostasis management range from the preemptive transfusion, in which blood products are transfused before laboratory abnormalities are recognized to a targeted therapy using purified coagulation factors and/or specific procoagulant drugs. What makes this management even more complex is the fact that the underlying rationale for starting coagulation therapy might be either completely empiric, or based on standard lab tests (which are sometimes time-consuming such that empiric therapy has already started before results are available). In addition, there is actually strong evidence that avoidance of exposure to allogeneic blood transfusion is of high importance, as it has been demonstrated to be associated with serious adverse events, such as acute lung injury, volume overload, nosocomial infections and sepsis, immunomodulation and organ dysfunction (14). In our experience viscoelastic tests [thromboelastography (TEG) or thromboelastometry (ROTEM)] were recently introduced to intraoperatively assess the clot formation and strength. These methods are useful to promptly diagnose acquired coagulation disturbances with the possibility to rapidly identify and correct specific defects in hemostasis and with a consequent significant reduction in blood transfusion and associated hospitalization costs (15).
The patient is maintained intubated and sedated during the warming time after the surgical procedure. In case of significant facial edema, the dual lumen endotracheal tube is not exchanged until an adequate edema reduction for fear of losing control of the airway. A chest X-ray is obtained immediately postoperatively to control pulmonary re-expansion and possible effusions. CSFD is performed in order to maintain a CSF pressure less than 10 mmHg and mean arterial pressure is forced above 90 mmHg in order to optimize SC perfusion. When the body temperature reaches at least 36 °C degrees sedatives are temporarily discontinued to assess neurological functions with the possibility to perform additional maneuvers in case of rapid onset SCI such as increased CSFD, forced hypertension and intravenous corticosteroids. As soon as the patient reaches hemodynamic and a respiratory stability the weaning from the ventilator is initiated. In our experience this approach resulted in significant improvements in neurologic function.
In uncomplicated cases, drainage tubes are removed at 36 to 48 hours postoperatively, while the intrathecal catheter of cerebrospinal fluid drainage is usually removed only after 72 hours. After respiratory weaning and endotracheal tube removal, non-invasive ventilation is performed in all the patients without specific contraindications with better respiratory and general outcome in our experience. In case of severe chronic kidney disease, transient temporary hemodialysis may be needed early after operation.
Results
In the last decade, a significant improvement in TAAA open repair results was observed with decreased morbidity and mortality rate compared with the first procedures performed over 40 years ago. Several advancements in preoperative diagnostic process, surgical technique, and postoperative care have been responsible for these improved results with SCI rates and 30-day mortality rates less than 10% reported in high volume centers (16). Nonetheless, despite this progress, mortality, neurologic injury, and renal dysfunction rates are still greater than desirable with an incidence of paraplegia reported in the literature that varies from 5% to 20% and with renal failure rates of 5% to 30%. The incidence of these devastating complications varies according to extent of the aneurysm, cross- clamp times, age of the patient, and comorbid conditions.
As described previously, several adjuncts have been introduced to decrease the incidence of complication after TAAA open repair and nowadays a multimodal approach is advocate. SC protection can be improved by CSFD, distal aortic perfusion, reattachment of intercostal arteries, intraoperative monitoring of MEP and SSEP, mild hypothermia, and intravenous corticosteroids. Visceral malperfusion rates are minimized with an adequate distal and direct perfusion. Renal function can be optimized with an adequate preoperative hydration, administration of mannitol and furosemide during the surgical procedure and in the postoperative period with an adequate perfusion obtained with endarterectomy, bypass or direct stenting in case of associated occlusive disease. Cold perfusion of the kidneys is also useful, and many authors have reported improved results using this technique. The best solution to be used is still under investigation.
Conclusions
The results of open surgical treatment of TAAA are improving with reduced postoperative mortality and morbidity. However, SCI, renal and visceral ischemic complications remain a not completely solved problem. A multimodal approach tailored to the specific risk factors of each patient is advocate to reduce postoperative complications. New protective strategies are still needed to further minimize the risks associated with TAAA repair.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Di Bartolomeo, Davide Pacini and Mohamad Bashir) for the series “Special Edition on The 9th Postgraduate Course on ‘Surgery of The Thoracic Aorta’ in Bologna” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.06.06). The series “Special Edition on The 9th Postgraduate Course on ‘Surgery of The Thoracic Aorta’ in Bologna” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Johnston KW, Rutherford RB, Tilson MD, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 1991;13:452-8. [Crossref] [PubMed]
- Spagnolo P, Giglio M. Role of Cardiac CT in assessment of patient with thoraco-abdominal aortic aneurysm. In: Chiesa R, Melissano G, Zangrillo A, et al. editors. Thoraco-Abdominal Aorta: Surgical and Anesthetic Management. Trento, Italy:.Springer-Verlag Italia, 2011:Cap 14, 173-82.
- Chiesa R, Melissano G, Civilini E, et al. Video-atlas of open thoracoabdominal aortic aneurysm repair. Ann Cardiothorac Surg 2012;1:398-403. [PubMed]
- Tshomba Y, Leopardi M, Mascia D, et al. Automated pressure-controlled cerebrospinal fluid drainage during open thoracoabdominal aortic aneurysm repair. J Vasc Surg 2017;66:37-44. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Civilini E, Melissano G, Chiesa R. Improved cannulation: technique for thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2010;89:675. [Crossref] [PubMed]
- Coselli JS, Tsai PI. Motor evoked potentials in thoracoabdominal aortic surgery: CON. Cardiol Clin 2010;28:361-8. [Crossref] [PubMed]
- Jacobs MJ, Mommertz G, Koeppel TA, et al. Surgical repair of thoracoabdominal aortic aneurysms. J Cardiovasc Surg (Torino) 2007;48:49-58. [PubMed]
- Tshomba Y, Kahlberg A, Chiesa R, et al. Comparison of renal perfusion solutions during thoracoabdominal aortic aneurysm repair. J Vasc Surg 2014;59:623-33. [Crossref] [PubMed]
- Kellum JA, Lameire NKDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. [Crossref] [PubMed]
- LeMaire SA, Jamison AL, Carter SA, et al. Deployment of balloon expandable stents during open repair of thoracoabdominal aortic aneurysms: A new strategy for managing renal and mesenteric artery lesions. Eur J Cardiothorac Surg 2004;26:599-607. [Crossref] [PubMed]
- Patel R, Conrad MF, Paruchuri V, et al. Balloon expandable stents facilitate right renal artery reconstruction during complex open aortic aneurysm repair. J Vasc Surg 2010;51:310-5. [Crossref] [PubMed]
- Chiesa R, Kahlberg A, Mascia D, et al. Use of a novel hybrid vascular graft for sutureless revascularization of the renal arteries during open thoracoabdominal aortic aneurysm repair J Vasc Surg 2014;60:622-30. [Crossref] [PubMed]
- Pieri M, Nardelli P, De Luca M, et al. Predicting the Need for Intra-operative Large Volume Blood Transfusions During Thoraco-abdominal Aortic Aneurysm Repair. Eur J Vasc Endovasc Surg 2017;53:347-53. [Crossref] [PubMed]
- Ghavidel AA, Toutounchi Z, Shahandashti FJ, et al. Rotational thromboelastometry in prediction of bleeding after cardiac surgery. Asian Cardiovasc Thorac Ann 2015;23:525-9. [Crossref] [PubMed]
- Wong DR, Parenti JL, Green SY, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. J Am Coll Surg 2011;212:569-79; discussion 579-81. [Crossref] [PubMed]
Cite this article as: Chiesa R, Rinaldi E. Thoracoabdominal aortic aneurysms open repair: a multimodal approach. J Vis Surg 2018;4:128.