Tracheal replacement: state of the art and novel perspectives
Immediate repair of long-segmental defects
Prosthetic tracheal repair
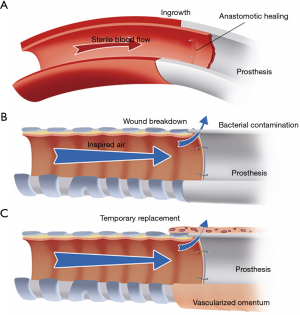
In recent years, most synthetic materials used for tracheal replacement have been tested in experimental animal research. From these studies, it became clear that definitive prosthetic replacement of the airway wall is not possible (1). To date, nearly all surgical prostheses that have been successful were observed in potentially sterile mesenchymal tissues. No example of successful prosthetic repair can be cited in the respiratory or gastrointestinal tract. The internal site of the airway tract belongs to the outside world, and bacterial contamination at the interface between the airway and prosthesis prevent its ingrowth (Figure 1). The complications of wound breakdown at the anastomoses can be temporarily delayed by wrapping the prosthesis in vascularized tissue, mostly transposed omentum.
Palliative treatment of long-segmental defects
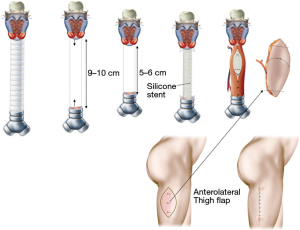
Long-segmental tracheal defects, which result after removal of malignant tumors are extremely rare. The only possibility for immediate reconstruction of these defects is to reduce the length of the defect by inserting a silicone stent, which is sutured to the upper and lower margins of the defect. A free fasciocutaneous skin tube (lateral thigh flap, radial forearm flap) can be used to wrap the silicone stent as a temporary closure (Figure 2) (2).
Tracheal transplantation
Introduction
Experience with tracheal allotransplantation has been anecdotal because of the difficulties linked with restoration of the blood supply. The first case of a tracheal allotransplant was reported in The Lancet in 1979 (3). Donor trachea was implanted heterotopically in the sternocleidomastoid muscle of the recipient and transferred to the orthotopic position 3 weeks later. However, the recipient was not given immunosuppressive therapy, no evidence of allograft viability was reported, and no information about the long-term outcome was published. The original article stated that “the tracheal allograft has become integrated and it has functioned perfect for 9 weeks without any evidence of rejection, ischemia, or infection.”
A second case of tracheal allotransplantation was reported in 1993 (4). The allotransplant was revascularized orthotopically under protection of immunosuppressive drugs. The graft appeared vital at the end of the second month, but signs of graft stenosis appeared by the end of the fourth month. However, the transplant was not visualized in the paper. Current knowledge suggests that orthotopic revascularization of a tracheal graft is completely impossible (Figure 3).
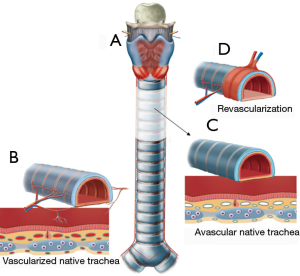
We began experimental animal research on tracheal allotransplantation in 1993 (5,6). In rabbits, the trachea was successfully transplanted in its orthotopic position after 2 weeks of heterotopical revascularization by wrapping in a vascularized fascia flap. From these studies, we learned that the trachea is subject to the same immunologic laws as all other allogeneic tissues. The most important component in tracheal rejection was lymphocyte-mediated, and the prime target cell population was the allograft endothelium (6). In 2008, we attempted tracheal allotransplantation in the clinic.
Learning curve of tracheal allotransplantation
In 2008, we were confronted with a difficult clinical case of long-segment stenosis. Tracheal allotransplantation was considered as a possible solution for the patients’ problem. Successful transplantation of a patch tracheal allograft was performed. The procedure involved the following key steps: (I) heterotopic revascularization of the cartilaginous trachea at the forearm under protection of immunosuppressive therapy; (II) replacement of the donor respiratory epithelium by recipient buccal mucosa; (III) withdrawal of immunosuppressive therapy; and (IV) orthotopic transplantation, with anastomosis of the radial vascular pedicle to blood vessels of the neck. Withdrawal of immunosuppressive drugs was possible because of the immune-privileged status of chondrocytes within the cartilage rings. As they are protected within a matrix, chondrocytes will remain vital if they are perfused by diffusion through recipient blood vessels from surrounding tissues (7,8).
Based on our experiences obtained in this first patient, we proposed the concept illustrated in Figure 4 for subsequent patients (8,9).
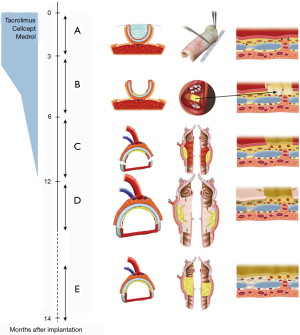
This concept was applied in six patients, including five patients with long-segment stenosis and one patient with a low-grade tracheal chondrosarcoma (9,10). The patient with chondrosarcoma was a 63-year-old man whose tumor developed over a period of more than 10 years. His airway was preserved by placement of a silicone stent. Due to stagnation of secretions, he required periodical bronchoscopic cleaning of the stent. However, he developed acute episodes of stent blockage, which made definitive treatment necessary. Four months after implantation of a suitable allograft in the left forearm (Figure 5), the tumor was resected through an anterior cervical incision with a sternotomy extension (Figure 6A,B,C,D). The tracheal allotransplant was used to repair the laryngotracheal defect.

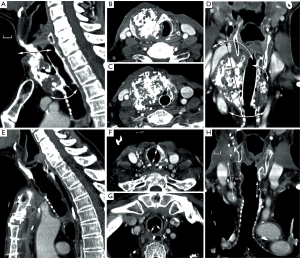
The potential for tumor progression while under immunosuppression for a low-grade malignancy was considered to be low. Computed tomography (CT) scan at the time of orthotopic transplantation demonstrated a nearly unchanged tumor bulk. Immunosuppressive medication was gradually phased out 11 to 12 months after orthotopic transplantation. The morphology of the transplant remained intact after withdrawal of immunosuppressive therapy (Figure 6E,F,G,H). Thus, it seems that mucosal repopulation of the transplant after cessation of immunosuppressants can occur with minimal loss of airway lumen.
Optimal tracheal allotransplantation concept
The major drawback of this transplantation concept is the long period of donor mucosal revascularization and regeneration. It takes 2 to 3 months before full mucosal regeneration is established. Moreover, both edges of the transplant are at risk of necrosis, with loss of some of the circumference of the cartilaginous trachea. In one of our patients, we made the seminal observation that the revascularization phase could be shortened from 2–3 months to 2 weeks when the luminal surface of the transplant was covered with well-vascularized tissue (Figure 7).
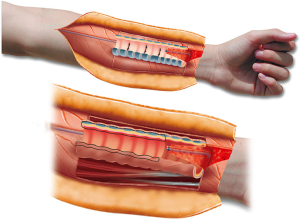
Our observation of the importance of covering the luminal site of the transplant with well-vascularized tissue led us to adapt our original transplantation concept. In subsequent patients, our goal has been to cover the luminal surface of the transplant as much as possible with the distal part of the radial forearm fascia flap (Figure 8).

Conclusions
We were the first to report a successful orthotopic allotransplant in 2010, using a novel protocol involving a controlled process of progressive vascularization, rejection of allogeneic mucosa, and preservation of the viability of the cartilage, which is otherwise immune-privileged. Our observations suggest that this unique vascularized transplantation technique with temporary immunosuppression generates a chimeric trachea, and that the immune-privileged nature of the cartilage has a central role of this process (7,8). The technique holds great promise for patients needing extensive airway reconstruction because the chimeric trachea graft does not require ongoing immunosuppression, a highly desired but elusive goal in the field of allotransplantation.
This technique was developed on the basis of animal research (6,7) and autotransplantation experience (11-13). It was further refined in a series of six patients (9,10). Through our experiences, we learned that vascular induction of the submucosal blood supply during graft revascularization is a different process from true angiogenesis (Figure 3) (9). Vascular induction of donor mucosal capillaries by recipient blood vessels around the transplanted tissue may occur through the intercartilaginous ligaments. In contrast, true angiogenesis with repopulation of the submucosal space by ingrowing recipient blood vessels is only possible in areas where the recipient blood vessels are in direct contact with the submucosal layer of the graft. Therefore, the most important adaptation was to allow for growth of recipient blood vessels in the submucosal space of the graft. This growth could be guaranteed by making partial incisions through the intercartilaginous ligaments. These incisions disrupted the barrier for angiogenetic outgrowth of recipient vessels and enabled ingrowth of recipient vessels into the submucosal space of the transplant (9).
Another important factor was the implantation of a recipient buccal mucosal graft in the central portion of the transplant (Figure 3). Buccal mucosa was chosen because respiratory mucosa is difficult to handle as a free graft. After ceasing immunosuppressive therapy, all donor respiratory epithelium will disappear, and the buccal mucosal graft will progressively grow and recover part of the surrounding transplant’s inner lining. At transplant sites lined with nonciliated squamous epithelium, the loss of mucociliary clearance will be compensated through coughing.
With the intercartilaginous incisions and the recipient buccal mucosa, immunosuppressive medication could be safely tapered and stopped 9 to 12 months after forearm implantation (9). Cartilage tissue escaped immunologic rejection owing to the absence of blood vessels and the protection of chondrocytes within a matrix (8). After cessation of immunosuppressive therapy, the surviving recipient mucosal graft will allow for secondary healing of the necrotic areas of donor epithelial lining (9).
The trachea is transplanted as a composite tissue, but the cartilage structure is the critical functional element of the graft. Cartilage is avascular, relies on indirect nutrition from the surrounding tissues, and is well known to be immune-privileged (8). The ingenious revascularization procedure, along with carefully timed immunosuppression, takes advantage of these unique properties so as to preserve the tracheal cartilage tissue and structure, while noncartilaginous tissues are replaced by recipient tissues.
Going forward, our principal objective is to optimize the revascularization process, thereby reducing the heterotopic grafting and immunosuppression phases. Specifically, instead of using fibrin glue, we plan to cover the luminal site of the heterotopic trachea with the distal part of the radial forearm fascia flap.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Federico Rea) for the series “Tracheal surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.08.05). The series “Tracheal surgery” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grillo HC. Tracheal replacement: a critical review. Ann Thorac Surg 2002;73:1995-2004. [Crossref] [PubMed]
- Beldholm BR, Wilson MK, Gallagher RM, et al. Reconstruction of the trachea with a tubed radial forearm free flap. J Thorac Cardiovasc Surg 2003;126:545-50. [Crossref] [PubMed]
- Rose KG, Sesterhenn K, Wustrow F. Tracheal allotransplantation in man. Lancet 1979;1:433. [Crossref] [PubMed]
- Levashov YuN, Yablonsky PK, Cherny SM, et al. One-stage allotransplantation of thoracic segment of the trachea in a patient with idiopathic fibrosing mediastinitis and marked tracheal stenosis. Eur J Cardiothorac Surg 1993;7:383-6. [Crossref] [PubMed]
- Delaere PR, Liu ZY, Hermans R, et al. Experimental tracheal allograft revascularization and transplantation. J Thorac Cardiovasc Surg 1995;110:728-37. [Crossref] [PubMed]
- Delaere PR, Liu Z, Sciot R, et al. The role of immunosuppression in the long-term survival of tracheal allografts. Arch Otolaryngol Head Neck Surg 1996;122:1201-8. [Crossref] [PubMed]
- Delaere P, Vranckx J, Verleden G, et al. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med 2010;362:138-45. [Crossref] [PubMed]
- Sykes M. Immune evasion by chimeric trachea. N Engl J Med 2010;362:172-4. [Crossref] [PubMed]
- Delaere PR, Vranckx JJ, Meulemans J, et al. Learning curve in tracheal allotransplantation. Am J Transplant 2012;12:2538-45. [Crossref] [PubMed]
- Delaere PR, Vranckx JJ, Den Hondt M, et al. Tracheal allograft after withdrawal of immunosuppressive therapy. N Engl J Med 2014;370:1568-70. [Crossref] [PubMed]
- Delaere P, Goeleven A, Poorten VV, et al. Organ preservation surgery for advanced unilateral glottic and subglottic cancer. Laryngoscope 2007;117:1764-9. [Crossref] [PubMed]
- Delaere PR, Vranckx JJ, Dooms C, et al. Tracheal autotransplantation: guidelines for optimal functional outcome. Laryngoscope 2011;121:1708-14. [Crossref] [PubMed]
- Loos E, Meulemans J, Vranckx J, et al. Tracheal Autotransplantation for Functional Reconstruction of Extended Hemilaryngectomy Defects: A Single-Center Experience in 30 Patients. Ann Surg Oncol 2016;23:1674-83. [Crossref] [PubMed]
Cite this article as: Delaere P. Tracheal replacement: state of the art and novel perspectives. J Vis Surg 2018;4:168.