3D photography method using digital subtraction angiography for detecting leakage point in a patient with pneumothorax
IntroductionOther Section
Secondary spontaneous pneumothorax (SSP) affects patients with underlying pulmonary disease, mainly chronic obstructive pulmonary disease (COPD). SSP is considered to differ from primary spontaneous pneumothorax (PSP) because of the life-threatening symptoms, the various locations of ruptured bullae, and the high rates of recurrence and mortality (1). Video-assisted thoracoscopic surgery (VATS) often reveals multiple bullae or diffuse pleural adhesions in the thoracic cavity, requiring the affected lung to be collapsed, and often making it difficult to find the leakage point (2). We have performed pleurography prior to surgery in order to find the leakage point, but as 2D images demonstrate perspective in only one direction, this is often impossible. We have therefore developed a system that can perform 3D photography with digital subtraction angiography (DSA), allowing detection of leakage points as air bubbles that can be confirmed in 3D. Herein, we report a patient in whom the leakage point was detected successfully by preoperative 3D photography with DSA, allowing thoracoscopic surgery to be performed.
Case presentationOther Section
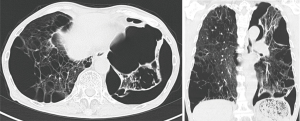
A 77-year-old man with COPD and left-sided SSP underwent VATS bullectomy of the lingular segment due to emphysema. At surgery, resected area was reinforced by covering with a glycolic acid/lactic acid polyester mesh (Vicryl Mesh® ETHICON). Two months later, the patient was hospitalized again for recurrence of SSP. CT scan on readmission demonstrated pneumothorax and several pleural adhesions of the left lung (Figure 1). A chest drainage tube was placed in the thoracic space. As surgical intervention for continuous air leakage seemed unavoidable, we selected 3D photography with DSA for detection of the leakage point before surgery, as detection by 2D pleurography proved impossible. After infusion of contrast medium from the drain into the thoracic cavity, 3D photography with DSA was performed over 200 degrees for 8 seconds (Figure 2). We were able to visualize bubbles from the dorsal side of the lower lobe near the spine, indicating the leakage point. We performed thoracoscopic surgery via a single 3-cm incision just above the leakage point, which revealed leakage from ruptured bullae in the lower lobe near the spine same, corresponding to the feature confirmed by preoperative 3D photography. Since there were several pleural adhesions at the surroundings of the bullae, less commonly we performed ligation of the bullae in the lower lobe without having to detach the pleural adhesions unnecessarily. The chest tube was removed on the day after surgery, and six months later there had been no recurrence of SSP.

CommentsOther Section
Patients with SSP due predominantly to chronic diffuse lung disease often have poor lung function and pleural adhesion, making surgery difficult. Detection of the leakage point before surgery may make VATS feasible, thus shortening the operation time and simplifying the procedure.
Pleurography can be used for detection of air leakage and can indicate the location, number and size of the bullae (4,5). However, use of this technique is not widespread, and requires a high level of examiner skill (5). Preoperative 3D photography with DSA may help to visualize the leakage point stereoscopically. Furthermore, both the size and degree of air leakage may be assessed in detail, thus helping surgery. In conclusion, we have successfully detected leakage points using preoperative 3D photography with DSA, allowing us to perform appropriate surgery.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.08.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Haga T, Kurihara M, Kataoka H. Spontaneous pneumothorax with persistent air leakage and invasive procedures. Intern Med 2013;52:2189-92. [Crossref] [PubMed]
- Yoshida C, Misaki N. 3D photography with DSA demonstrated bubbles from the dorsal side of the lower lobe near the spine, indicating the leakage point. Asvide 2018;5:735. Available online: http://www.asvide.com/article/view/26960
- Bölcskei PL, Haberstumpf H, Trapp VE, et al. Applications and uses of pleurography in patients with spontaneous pneumothorax. Pneumologie 1990;44:186-7. [PubMed]
- Kurihara M, Kataoka H, Ishikawa A, et al. Latest treatments for spontaneous pneumothorax. Gen Thorac Cardiovasc Surg 2010;58:113-9. [Crossref] [PubMed]
Cite this article as: Yoshida C, Misaki N. 3D photography method using digital subtraction angiography for detecting leakage point in a patient with pneumothorax. J Vis Surg 2018;4:187.