The frozen elephant trunk technique for treatment of thoracic aortic aneurysm with Japanese-made open stent graft: FROZENIX
Introduction
Recent advancement of endovascular treatment for the thoracic aortic disease has widened surgical indications because of less invasiveness and has made the surgical procedure simpler as well. Frozen elephant trunk (FET) procedure has emerged in order to facilitate distal lumen thrombosis around the graft but also easier anastomosis at the distal aortic anastomosis during total arch replacement (1,2). Several commercials made FET graft currently available are showed favorable results (3-5). Japan-made FET, FROZENIX, was launched 2014 and since then, more than 6,000 prostheses have been implanted. Main advantages of FROZENIX are easier handling and implantability, and a better fitting comfortability to the curvature of the aortic arch. This paper presents a representative case.
Case presentation
The patient was a 79-year-old male who was diagnosed to have a fusiform aneurysm located in the distal arch and 1-year-old chronic type B aortic dissection. Physical and laboratory examination showed no abnormality. Echocardiography and coronary angiography were normal. The Japan score and EuroScore II was 6.9 and 8.7, respectively. An arch aneurysm was 53 mm in diameter and contained thick mural thrombi. The diameter of the descending aorta was 42 mm. Total arch replacement was conducted in the following sequence: The arterial cannula was inserted in the ascending aorta, cerebral protection using moderate hypothermic circulatory arrest combined with selective antegrade cerebral perfusion (ACP), insertion of the FET (FROZENIX®, Japan Life Line, Tokyo), distal arch reconstruction, proximal arch reconstruction, and finally epi-aortic vessel re-implantation. The consent was taken at the admission.
Video (Figure 1)

Preoperative 3D CT scan showed a fusiform aneurysm existed distally to the left subclavian artery. The diameter of an aneurysm was 60 mm and there were rich mural thrombi.
After the routine mid-sternotomy, the innominate vein was fully mobilized. Dissection of an aneurysm, aortic arch branches, or the vagal nerve was never tried. No taping around the arch or arch vessels was applied. The cardiopulmonary bypass was established by the ascending aorta cannulation, arterial cannula (20 Fr DLP, Medtronic, Minneapolis, USA) was inserted in the ascending aorta where no or little atheromatous change was found by direct echo scanning. Two venous cannulae (Medtronic, Minneapolis, USA) were inserted from the right atrium into the superior vena cava and to the inferior vena cava. Core cooling was done until both tympanic temperatures were down to the 23 °C and the rectal temperature to the 30 °C. During cooling, a 0.035-inch guiding wire (Radifocus®, Terumo, Tokyo) was introduced in the descending aorta from the left femoral artery under a guidance of the transesophageal echography (TEE). The transcranial motor evoked potential of the spinal cord (MEP) and the drainage of the cerebrospinal fluid (CSFD) were monitored. Three traction sutures at the root of the arch vessels and one at the lesser curvature of the arch were liberally used to facilitate exposure.
After the tympanic temperature reached to 23 °C and rectal temperature below 30°C, the total circulatory arrest was done with CVP rising to 5 mmHg. The heart was arrested by the retrograde cardioplegia. The aortic cannula was removed and the aortic arch was opened longitudinally. Two balloon-tipped cannulae (Sumitomo Bakelite, Akita) were inserted to the left common carotid artery (12 Fr) and the brachiocephalic artery (16 Fr) from inside of the arch. After full venous drainage was achieved, the left subclavian artery was transected at its origin and a 12 Fr cannula was inserted. These cannulae were fixed to the skin right side and the ACP was started using a single roller pump with a flow of 10 to 12 mL/kg/min. The perfusate temperature was kept to 23 °C. The left and the right radial pressure, as well as line pressure of the balloon catheters, was monitored to control the pressure between 30 to 50 mmHg. Cerebral oxygen saturation (rSO2) was monitored bilaterally in the forehead using near-infrared spectroscopy. The aortic arch was transected between the left common carotid artery and the left subclavian artery. Proximal stump of the left subclavian artery was oversewn using a buttressed 3-0 polypropylene suture (Prolene®, Ethicon, USA).
A FET (J graft Frozenix® 33 mm × 120 mm, Japan Life Line, Tokyo) was prepared. A gentle curve was made at the stent-graft portion of the FET according to the shape of the aortic arch to the descending aorta. The guide wire was introduced in the central lumen of the FET. The descending aorta was filled with blood and the FET was introduced in the true lumen of the descending aorta. The marker of the FET was set to the proximal edge of the aortic arch. The FET was deployed by pulling the sheath proximally with monitoring the TEE. The sheath of the FET, guiding rod, and a guiding wire was removed. A flexible portion of the FET was resected and the FET was fixed at the arch with Teflon felt reinforcement using 3 mattress 4-0 polypropylene sutures (Nespilene®, Alfresa, Tokyo).
A 26 mm 4-branched Dacron graft (J graft®, Japan Life Line, Tokyo) was anastomosed to the stump of the aortic arch, incorporating FET and the Teflon felt using a long 4-0 monofluorvinylydene suture (Monofulene® 120 cm, 22 mm needle; Alfresa, Japan). The flexible sucker was placed inside the graft to suck the blood in the descending aorta. Initially, at 9 o’clock, the suture was placed in the graft, the FET, aorta, and the Teflon strip. After tying down, the suture went counter clockward. Initially, the needle was carried by forehand and formed the 3 o’clock direction of the needle was changed to a reverse manner. The suture was tied at 9 o’clock. After the flexible sucker was removed and the descending aorta was filled with blood, antegrade perfusion of the lower body was slowly started through the 4th side branch of the graft. Checking the anastomosis, then rewarming was started. Coincident with re-warming, antegrade SCP flow was gradually increased while maintaining the baseline values of rSO2. However, antegrade SCP flow was limited below 1,200 mL/min to prevent brain edema.
The ascending aorta was transected 2–3 cm above the ST junction. A Teflon strip was wrapped around the aorta and proximal anastomosis to the aorta was done using a 4-0 polypropylene suture (Nespilene® 120 cm, 17 mm needle; Alfresa, Japan). After de-airring procedure, the graft clamp was released and the heart was reperfused. Usually, a spontaneous beating of the heart was obtained soon.
Aortic arch was divided to make the arch vessel buttons with each traction suture. Three arch vessels were reconstructed tandem to the graft branches using a 5-0, 17mm needle polypropylene suture (Nespilene®, Alfresa, Japan). The balloon cannula was removed near the end of each anastomosis and liberal backflow was used for flushing the debris. Usually, the parachute technique was used and each vessel was perfused after completion of the anastomosis.
Then the CPB was weaned-off. The left pleura were opened longitudinally near the sternum to monitor unexpected bleeding into the left pleural cavity.
Duration of the CPB was 110 minutes, cardiac ischemic time 37 minutes, duration of the circulatory arrest of the lower body 22 minutes, duration of the ACP 63 minutes. The minimum temperature of the tympanic membrane and rectum was 22.0 °C and 26.2 °C, respectively. The postoperative course was straightforward. He was extubated on the same day. Postoperative CT scan showed a satisfactory result.
Discussion
In 1983, Borst et al. introduced the two-stage elephant trunk principle into the strategy of surgical treatment for an extensive thoracic aortic disease (7). This approach is based on the prosthetic replacement of the whole arch with an elephant trunk extension of the arch graft inserted into the descending aorta during the first-stage operation performed through a median sternotomy. However, the graft segment forming the elephant trunk is free floating in the descending aortic lumen, thus impeding thrombus formation between the graft and the aneurysmal vessel wall. This method intrinsically requires the second stage operation through a left thoracotomy. In 1996, Kato et al. (1) first reported 10 patients who had undergone home-made stent-graft insertion in the descending aorta or in the true lumen of the descending aorta. All patients had a mid-sternotomy and cardiopulmonary bypass under deep hypothermia was applied. Their stent-graft was constructed with a self-expanding (Gianturco®) stent that is anchored into the woven Dacron graft (UBE®, Japan). Complete thrombosis of the aneurysms or false lumens surrounding the grafts resulted in all 10. They showed a possibility where the future left thoracotomy is not required in these patients. They named this method “open stent grafting”. In 2003, Karck et al. (2) reported 4 patients with aneurysms of the descending aorta or chronic aortic dissection who had an open aortic arch replacement with stent-graft insertion on the descending aorta. They used a custom-made stent graft (Chavan-Haverich®, Curative Medical Devices Gmbh, Dresden). They found a complete thrombosis in the descending aorta or false lumen around the stent-graft. This was the first report where this method was called as “frozen elephant trunk (FET)”. Since then, the FET procedure has been widely spread mainly in Europe and many clinical investigations have been reported using commercial-made products (4,5,8-13). This procedure virtually excluded the necessity of surgical replacement of the descending aorta and majority of the patients who had FET at the first stage had the subsequent endovascular stent-graft insertion in the descending aorta. However, not all patients showed a decrease in the size of the aneurysm of the descending aorta and problems in the thoracoabdominal aortic portion are not solved by this technique.
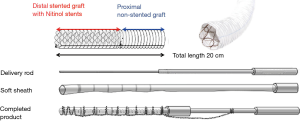
A Japan-made FET prosthesis (J graft Frozenix®, Japan Life Line, Tokyo) was first launched in 2014. The prosthesis consisted of a Dacron polyester fabric vascular prosthesis with Nitinol stents affixed on the inner aspect. The Nitinol stent was hand-knitted not to change length as the diameter change. All stents were fully covered with the Dacron prosthesis. The proximal portion of the FROZENIX stent graft was composed of a simple Dacron graft. The Dacron graft had external velour with a thickness of 300 micro mm and its water porosity was 150 mL/cm2. The delivery system consisted with a malleable rod (10 Fr) and could be advanced into the descending aorta over a 0.035-inch flexible guide wire the stent-graft. The size of the FROZENIX stent graft was chosen to match the diameter of the descending aorta or the diameter of the true lumen. The system was wrapped by a smooth-surfaced polyester mesh. The sheath had markers of 1 cm-interval in the non-stented portion and the last marker should be pointed at the edge of distal aorta. Because the stent did not change its length throughout, the distal end of the stent graft can be fixed as expected. Withdrawal of the outer sheath while the inner rod was held steady released the stented portion of the FROZENIX stent graft. The proximal Dacron tube could then be released by simply pulling back both the sheaths and rod. Any balloon modulation to accommodate the stent graft to the descending aorta was necessary. The products had a range of diameter from 21 to 39 mm (2 mm step) and the length of the stented portion was 60, 90 and 120 mm. The total length of the stent graft was 200 mm in all. The length of the total system was 57 cm and 12 mm in diameter (Figure 2).
Clinical application of the FROZENIX covers a wide spectrum of the thoracic aortic disease, including extensive arch-descending aneurysm and acute or chronic DeBakey type I aortic dissection (14). Some studies comparing the patency of the false lumen in the descending aorta after replacement of the ascending aorta or total arch for acute type I aortic dissection showed a higher rate of false lumen thrombosis in total arch replacement group. There is no consensus about the superiority of the FET over the classical free-floating elephant trunk. However, less incidence of surgical bleeding from the suture line and fewer anastomotic leakage into the false lumen is expected in patients who had FET. Current our standard method of total arch replacement in acute type I aortic dissection is set the aortic anastomotic site at between the left common carotid artery and the left subclavian artery. We liberally use a FROZENIX graft and a zero-porosity four branch Dacron graft under the ACP.
The main concern of this method is a relatively higher incidence of development of spinal cord ischemia (15). The clinical trial of the FROZENIX implanted in 60 patients disclosed that four patients (6.7%) had spinal cord ischemia. A recent survey showed an improved incidence of spinal cord ischemia as 1.7% in subsequent 4,600 patients. Deliberate deployment of the distal stent-graft in the descending aorta above the level of the aortic valve should be warranted to prevent spinal cord complications (16).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Di Bartolomeo, Davide Pacini and Mohamad Bashir) for the series “Best Video Presentation Prize for the 9th Postgraduate Course - Surgery of the Thoracic Aorta” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.08.18). The series “Best Video Presentation Prize for the 9th Postgraduate Course - Surgery of the Thoracic Aorta” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kato M, Ohnishi K, Kaneko M, et al. New graft-implanting method for thoracic aortic aneurysm or dissection with a stented graft. Circulation 1996;94:Ii188-93. [PubMed]
- Karck M, Chavan A, Hagl C, et al. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg 2003;125:1550-3. [Crossref] [PubMed]
- Di Bartolomeo R, Di Marco L, Armaro A, et al. Treatment of complex disease of the thoracic aorta: the frozen elephant trunk technique with the E-vita open prosthesis. Eur J Cardiothorac Surg 2009;35:671-5; discussion 675-6. [Crossref] [PubMed]
- Jakob H, Dohle D, Benedik J, et al. Long-term experience with the E-vita Open hybrid graft in complex thoracic aortic diseasedagger. Eur J Cardiothorac Surg 2017;51:329-38. [PubMed]
- Czerny M, Barchichat I, Meszaros K, et al. Long-term results after proximal thoracic aortic redo surgery. PLoS One 2013;8:e57713 [Crossref] [PubMed]
- Okita Y. Total arch replacement with the frozen elephant trunk. Asvide 2018;5:739. Available online: http://www.asvide.com/article/view/26974
- Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using "elephant trunk" prosthesis. Thorac Cardiovasc Surg 1983;31:37-40. [Crossref] [PubMed]
- Di Bartolomeo R, Cefarelli M, Folesani G, et al. Frozen elephant trunk surgery using the Vascutek Thora-flex hybrid prosthesis. Ann Cardiothorac Surg 2013;2:660-2. [PubMed]
- Leontyev S, Tsagakis K, Pacini D, et al. Impact of clinical factors and surgical techniques on early outcome of patients treated with frozen elephant trunk technique by using EVITA open stent-graft: results of a multicentre study. Eur J Cardiothorac Surg 2016;49:660-6. [Crossref] [PubMed]
- Luehr M, Etz CD, Mohr FW, et al. Surgical management after stent-graft failure during the frozen elephant trunk technique for acute type A aortic dissection. J Thorac Cardiovasc Surg 2012;144:e106-8. [Crossref] [PubMed]
- Ma WG, Zhu JM, Zheng J, et al. Sun's procedure for complex aortic arch repair: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:642-8. [PubMed]
- Roselli EE, Soltesz EG, Mastracci T, et al. Antegrade delivery of stent grafts to treat complex thoracic aortic disease. Ann Thorac Surg 2010;90:539-46. [Crossref] [PubMed]
- Tian DH, Wan B, Di Eusanio M, et al. A systematic review and meta-analysis on the safety and efficacy of the frozen elephant trunk technique in aortic arch surgery. Ann Cardiothorac Surg 2013;2:581-91. [PubMed]
- Easo J, Weigang E, Holzl PP, et al. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection: analysis of the German Registry for Acute Aortic Dissection Type A. J Thorac Cardiovasc Surg 2012;144:617-23. [Crossref] [PubMed]
- Usui A, Fujimoto K, Ishiguchi T, et al. Cerebrospinal dysfunction after endovascular stent-grafting via a median sternotomy: the frozen elephant trunk procedure. Ann Thorac Surg 2002;74:S1821-4; discussion S1825-32.
- Flores J, Kunihara T, Shiiya N, et al. Extensive deployment of the stented elephant trunk is associated with an increased risk of spinal cord injury. J Thorac Cardiovasc Surg 2006;131:336-42. [Crossref] [PubMed]
Cite this article as: Okita Y. The frozen elephant trunk technique for treatment of thoracic aortic aneurysm with Japanese-made open stent graft: FROZENIX. J Vis Surg 2018;4:188.