
Thymectomy for thymoma spanning three decades at Tor Vergata thoracic surgery center
Introduction
In the last decades increasing attention has been attributed to thymomas considered a neoplasm with evident or potential malignant characteristics. They represent the most common primary anterior mediastinal neoplasm in adult and complete surgical removal is still considered the best therapeutic option (1-6).
To date, controversies still exist about the best way for surgical approach to the tumor, the adequacy of extent resection, the best sequence of therapeutic strategy as well as the most reliable prognostic indicators. Our series of thymectomy for thymoma dates several decades. Along this period we approached thymomas through different surgical routes yet always aiming at the complete removal of the tumor together with the whole thymic gland, periglandular fatty tissue and involved adjacent structures as well. We investigated the biological prognostic factors predicting long-term survival and postoperative recurrence. Hereby, we retrospectively reviewed our long experience analyzing long-term outcomes.
Methods
Patients
This is a retrospective, chart Institutional Review Board authorized, analysis of all patients who underwent thymectomy for thymoma in different centers by a single surgeon (TCM) from 1986 to 2015.
Procedures were carried out at the Thoracic Surgery of St. Eugenio Hospital of Rome [1986–2002] and the Thoracic Surgery Division of the Policlinic Tor Vergata University of Rome [2002–2015]. In addition, operations performed in private hospitals were also included.
The Tor Vergata Foundation Institutional Review Board authorized the revision of the charts. Due to the long time period considered and the different clinical institutions involved permission was issued with waiver of informed consent from the uncontactable patients (PTV No. 2017-187-3).
According to these bases we enrolled in the study a total of 225 patients operated on extended resection of thymoma with total thymectomy in all disease stages. Demographic and clinical data are resumed in Tables 1 and 2. All patients routinely underwent studies to detect incidentally associated myasthenia gravis (MG) that included single-fiber electromyography and acetylcholine-receptor antibodies dosage ethical statement.
Table 1
| Variables | Thymoma (n=225) | Thymic carcinoma (n=17) | Carcinoid (n=7) | Thymolipoma (n=8) |
|---|---|---|---|---|
| Age (years) | 47.5±15.3 | 46.2±15.5 | 50.4±15.1 | 32.3±14.3 |
| Gender (M:F) | 127:98 (56%:44%) | 8:9 (47%:53%) | 3:4 (43%:57%) | 4:4 (50%:50%) |
| Myasthenia Gravis | 80 (35.6) | – | 1 (14.3) | – |
| Chest pain | 33 (14.7) | 16 (94.1) | 4 (57.1) | 2 (25.0) |
| Dyspnea | 29 (12.9) | 14 (82.4) | 3 (42.9) | 7 (87.5) |
| Venous obstruction | 22 (9.8) | 15 (88.2) | 1 (14.3) | – |
| Asymptomatic | 106 (47.1) | 1 (5.9) | 3 (42.9) | 1 (12.5) |
| Diameter (cm) | 5.51±3.25 | 7.31±4.25 | 5.21±1.38 | 14.38±5.61 |
| Operation time (min) | 165±43.7 | 158±71.2 | 143±41.2 | 125±41.7 |
| Intraoperative blood loss (mL) | 143±55.6 | 181±59.5 | 121±29.3 | 91±33.4 |
| Postoperative drainage (days) | 3.3±1.6 | 4.2±1.8 | 3.4±2.2 | 5.2±1.5 |
| Postoperative stay (days) | 5.2±1.3 | 6.9±1.0 | 3.3±1.4 | 4.4±1.4 |
| 30-day mortality | – | – | – | – |
| 30-day major morbidity | 13 (5.8) | 3 (17.6) | 1 (14.3) | 1 (12.5) |
| Complete resection | 189 (84.0) | 3 (17.6) | 6 (85.7) | 8 (100.0) |
| Recurrent cases | 55 (24.4) | 14 (82.4) | 1 (14.3) | – |
Table 2
| Variables | Total (n=225) | Open (n=182) | Minimally (n=43) | P |
|---|---|---|---|---|
| Age (years) | 47.5±15.3 | 46.2±15.5 | 50.4±15.1 | 0.19 |
| Gender (M:F) | 121:94 (56%:44%) | 106:76 (58%:42%) | 20:23 (46%:52%) | 0.09 |
| Myasthenia gravis, n (%) | 80 (35.6) | 68 (37.4) | 12 (27.9) | 0.3 |
| Masaoka stage, n (%) | 0.002 | |||
| I | 92 (40.9) | 64 | 28 | |
| II | 76 (33.8) | 66 | 10 | |
| III | 41 (18.2) | 39 | 2 | |
| IV | 16 (7.1) | 16 | – | |
| WHO classification, n (%) | 0.52 | |||
| A | 31 (13.8) | 23 | 8 | |
| AB | 76 (33.8) | 58 | 18 | |
| B1 | 67 (29.8) | 56 | 11 | |
| B2 | 33 (14.7) | 29 | 4 | |
| B3 | 18 (8.0) | 16 | 2 | |
| Diameter (cm) | 5.51±3.25 | 7.31±4.25 | 2.59±1.44 | 0.03 |
| Operation time (min) | 165±43.7 | 151±44.5 | 175±42.9 | 0.41 |
| Intraoperative blood loss (mL) | 143±55.6 | 153±54.5 | 113±25.4 | 0.03 |
| Postoperative drainage (days) | 3.3±1.6 | 4.1±1.6 | 1.5±1.1 | 0.02 |
| Postoperative stay (days) | 5.2±1.3 | 6.3±1.7 | 3.2±1.5 | 0.04 |
| 30-day mortality | – | – | – | – |
| 30-day major morbidity | 12 (5.3) | 10 (5.5) | 2 (4.7) | 0.06 |
| Complete resection | 189 (84.0) | 153 (84.1) | 36 (83.7) | 0.38 |
| Neoadjuvant therapy | 44 (19.6) | 34 (18.7) | 10 (23.3) | 0.81 |
| Adjuvant therapy | 84 (37.3) | 70 (38.5) | 14 (32.5) | 0.26 |
Surgery
Complete curative resection of the thymoma was always our main task whatever the surgical approach. Equally to non-thymomatous thymectomy also in thymomas the resection was as extended as possible including all mediastinal perimeter, likely site of thymic tissue, but also exploring lung and pleural cavities. In preparation for surgery all patients underwent chest computed tomography (CT) scan, magnetic resonance (MR), pulmonary function tests, appropriate cardiac assessment and preoperative blood work. Since 2005 positron emission tomography (PET) combined with CT was routinely introduced (7,8). Patients with MG were optimized in conjunction with referring neurologist. All MG patients underwent Ambrogi’s rehabilitation protocol (9). Needle biopsy were never performed. We always used the first Masaoka staging system (10).
When the tumor was massively extended we only adopted to de-bulk the mass. The involvement of mediastinal structures was anyway treated with adequate resection of mediastinal fat around the great vessels. All patients predicted at stage IV and someone at stage III preoperatively received induction chemotherapy in the hope of downstaging the disease.
Type of approach and resection were always discussed and chosen after panel discussion among specialists of oncology, radiotherapy, anesthesiology, intensive care, neurology, pulmonology and physiotherapy.
Total median sternotomy
The experience with sternotomy dated since one of us (TCM) was assistant professor at Thoracic Surgery Division of La Sapienza Rome University under the direction of Professor Costante Ricci, mentor and pioneer of thoracic surgery in Italy (11,12). This approach has been classically used for the management of the tumors of the anterior mediastinum and cardiac pathologies. For decades full sternotomy fulfilled all the requirements to treat thymomas at any stage. This approach was progressively abandoned given the increasing popularity of video-assisted thoracic surgery (VATS). However, transternal approach is still performed in invasive thymomas and whenever concomitant demolition and reconstructive procedures are required.
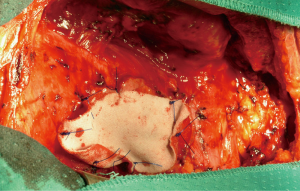
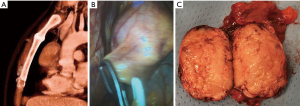
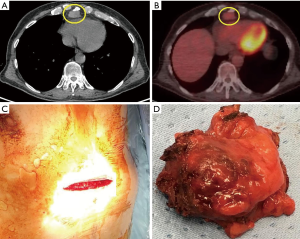
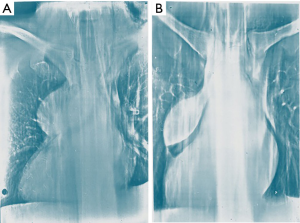
Transternal thymectomy was accomplished as usually described (13-16). As a rule transternal thymectomy entails total thymectomy for all Masaoka stages. Resection also includes all fat tissue in the anterior mediastinum from one phrenic nerve to the other and from the diaphragm to the bottom of the thyroid gland. The innominate axis is accurately skeletonized. Both pleural spaces are widely opened to permit complete exploration, evaluation of the extent of the tumor, as well as individuation of the phrenic nerves in order to prevent their damage unless directly involved by the tumor. Care should be taken to avoid damages to the phrenic nerve, but when it is incorporated within the tumor mass and complete resection is otherwise impossible, the nerve may be sacrificed. The en-bloc removal of the tumor and the gland is the primary goal avoiding capsule tears in order to limit potential disseminations and recurrences. The transternal approach facilitates the extent of resection and vessel reconstructions such as superior vena cava (Figure 1). Large pericardial defects are repaired by prosthetic mesh (Figure 2). Obviously, we observed all the amount of complications following sternotomy including partial sternal dehiscences. At the end of the operation mediastinal thymic bed is carefully inspected to be certain of both hemostasis and totality of thymectomy. In order to facilitate the pathologist’s task we usually collocate the main specimen and all fragments of resected fat tissue into a standard plastic cast mimicking the normal anatomy of the gland and its orientation in the mediastinal area. This expedient avoids the confusion that could emerge about the exact orientation of the tumor and resection margin status. In particular, in this map we highlight area adjacent to pericardium, innominate axis, superior vena cava, phrenic nerves and pleural cavities. This is very important to define the stage according to Masaoka, which we have routinely employed since the beginning of our experience. We have similarly transferred this setting in VATS and subxiphoid thymectomies.
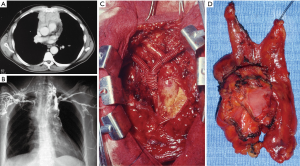
In some cases sternotomy was combined with lateral thoracotomy in order to facilitate the extended resection including pericardium, innominate vein, superior vena cava, lung and diaphragm, and all detectable implants in pleura.
VATS
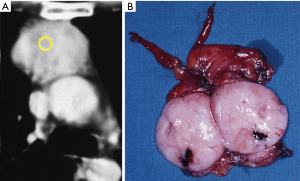
We used transternal approach until the advent of VATS. As a matter of fact, starting from the mid ’90s with the introduction of the video-assisted technologies, minimally invasive thymectomy took progressively place although majority of the procedures had been still done through open access due to supposed better neoplastic radicality. We have performed triportal VATS extended thymectomy for nonthymomatous MG since 1996 (17). Initially, we chose the left-side approach as described in our previous paper (18) and then also preferring the right-side according to the convenience (19). With the accrual of confidence in minimally invasive procedures, in 2005 we started a program of VATS extended thymectomy for small and early-stage thymomas (Figure 3). In performing VATS thymectomy we always followed the principles of minimally invasive resections of thymomas published by the International Thymic Malignancies Interest Group (20). VATS was usually approached through only one side chosen according to the prevalence side-location of the tumor as evidenced by imaging.
In the case of preoperative prediction or evidence at first exploration of involvement of the phrenic nerve, innominate vein or other major vessels, VATS procedure is contraindicated. However, video-assisted manipulation of the phrenic nerve by itself may be not difficult depending on the surgeon’s experience. Gentle retraction is mandatory and this is easier in less invasive tumor. If this is not feasible the opportunity of conversion to open access should be taken into account.
Left-sided VATS approach is used for large tumors. In these cases it may be useful to first remove the tumor and then the thymic gland. Dissection is facilitated by the use of novel energy devices.
After en-bloc removal of tumor and gland, the fat tissue lying in the left anterior mediastinum and cardiophrenic angle is removed. This is particularly important when MG is associated. If adherent to the thymic tumor the fat tissue in the aorto-pulmonary window must be removed having care to preserve the phrenic nerve, recurrent laryngeal nerve as well as pericardial-diaphragmatic vessels. When the pericardium is tightly connected to the tumor it must be opened 1 cm around the lesion and in front of the phrenic nerve in order to better protect the affected area and exclude myocardial involvement. If myocardium is involved conversion to thoracotomy becomes necessary in order to resect the lesion. The adherent pericardium should be removed. Small pericardial defects between 4–6 cm in diameter, are repaired under thoracoscopy by continuous cross-stitching using no.1 absorbable sutures. A Marlex mesh is utilized for larger defects in order prevent heart hernia.
Then, the right mediastinal pleura is widely opened to remove the fat tissue in the right anterior mediastinum and cardiophrenic angle. This is a very important step in myasthenic patients because pathologic examination of these areas can disclose ectopic thymic tissue sustaining persistent MG (21).
If necessary, in order to adequately control the controlateral side, we add an uniportal opposite side access, which allows the exploration of the opposite side and the incidental removal of deposits.
Mobilization of upper hornes of the thymus can be facilitated by a cervical port, 2 cm skin incision just above the sternal notch, used as an adjuvant access. The upper poles of the thymus are identified and mobilized similar to transcervical thymectomy. Then, they are placed in the superior mediastinum to be located during VATS. Despite the minimal invasiveness VATS permits the resection of limited invasion of neighboring structures such as innominate vein, lung and pleural deposits allowing similarly extensive resection than those performed through median sternotomy. Namely, innominate vein can be easily sutured by linear stapling device. During VATS dissection, particular care deserves capsule manipulation thus avoiding tears and potential pleural dissemination. Similarly to the resected neoplastic thymus, all the material dissected is placed in a specimen bag to be extracted out to limit the risk of local dissemination. Whenever necessary the removal of huge specimen can be feasible through a subxiphoid approach that we had largely employed in the surgery of lung metastases (22).
After having gained experience with the three-portal approach and whenever allowed by CT evidence of an early-stage thymoma we have lastly come to the uniportal approach with excellent results (Figure 4).
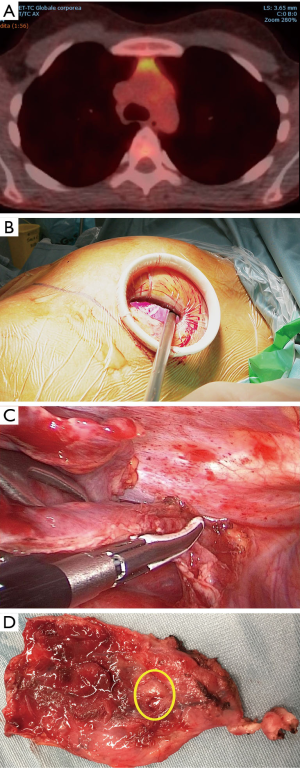
Subxiphoid VATS approach
Since 2010 we called at the subxiphoid VATS approach that has been successfully introduced with a consistent postoperative recovery yield. A 2.5–3.5 cm transverse skin incision is made 1–2 cm below the lower edge of the xiphoid (Figure 5). The anterior layer of the rectus sheath is cut and digitally dissected on the reverse side of the xiphoid. A plastic self-standing sleeve retractor (Alexis® Applied Medical, Amersfoort, Netherlands) is inserted and fixed. If necessary, additional bilateral transthoracic incision is added to facilitate lung and pleura deposits removal. The thymus is carefully dissected from caudal to cephalad avoiding capsule tears. A cervical incision is added whenever necessary both to facilitate cervical dissection of the upper thymic horns and to complete resection at this level. When closely connected with the tumor combined resection of the innominate vein can be safely executed through this route by dedicated stapling devices. At the end of operation all resected specimens are removed through the subxiphoid route, that can be enlarged according to the size of the tumor, using a dedicated plastic endobag. After the removal of the tumor the fat tissue in the anterior mediastinum and both cardio-phrenic angles is cleaned and extracted in same way.
Postoperative surveillance and follow up
Postoperatively MG patients were assisted in the thoracic surgery ward or, when necessary, in the intensive care unit. Patients with MG reintroduced their usual dosage preoperative medications immediately after surgery (23). Several precautions were recommended for a minimum of 6 weeks after sternotomy while no caution were suggested in VATS patients.
All patients after the surgical procedure patients underwent at least a CT scan of the chest every 6 months for the first two years and afterwards yearly. Adjuvant therapy with combined radio and chemotherapy was performed in the case of young subjects (less than 50-year-old) at stage II independently from resection margin finding and in all patients at stage III.
Follow up was performed by outpatient clinics and direct contact with the primary care physician. Recurrence represents the most critical discriminator in the follow up of long-surviving neoplastic disease and were divided into three categories. Local recurrences were defined as disease occurring in the bed of the thymus that is the anterior mediastinum or tissues immediately contiguous with the thymoma or normal thymus. Regional recurrences were defined as intrathoracic tumor that is not immediately contiguous with the thymus or the previous thymic tumor including parietal or visceral pleura and pericardial nodules, with exception of the area of resected thymoma. Distant recurrence was disease sited outside the thorax including lower neck (24).
Information regarding survival and clinical status were retrieved during outpatient sessions or by telephonic enquires. Second recurrence was identified after radical treatment of the first recurrence.
In the concomitance of MG the evolution of the disease was analyzed with the Osserman scale (25) and the Quantitative MG score (26) at timed interval. This last score was introduced starting from 2000 and entails a scale from 0 (no impairment) to 39 (maximal impairment) evaluated by an experienced neurologist belonging to our Myasthenia Unit. Evaluations prior to 2000 were retrospectively assessed with the same score according to clinical data of MG status. Prior to thymectomy we use to administrate a plasmapheresis cycle or intravenous immunoglobulin G in order to minimize the risk of thymectomy MG exacerbation and myasthenic crisis. All patients with MG underwent a structured pre and postoperative rehabilitation program according to our Myasthenia Unit guidelines (27).
Statistical analysis
Statistical analysis was performed using the SPSS package, version 20.0 (IBM, Armonk, NY, USA). Descriptive statistics was presented as mean and standard deviation. Due to the non-normal distribution of some variables, non-parametric tests for paired, and unpaired comparisons (Wilcoxon-sum rank and Mann-Whitney, respectively) were used. Significance was set at P<0.05. Overall and disease-free survivals were assessed with the Kaplan-Meier method starting from the time of the operation. The day of the operation was the initial point in both disease-free and overall survivals whereas the day of recurrence discovery and the day of last-follow up or death were the endpoints, respectively. Obviously, patients undergoing only incomplete resections were investigated only as far as overall survival is concerned. Due to the long-time of follow up also death of other cause were considered and kept separated from the evaluation. In addition also further disease free survival times after any redo surgery were evaluated. Prognostic factors evaluation was performed with the log rank test.
Also time to complete stable remission from thymectomy was estimated with the Kaplan-Meier method. Influence of thymoma on complete stable remission curve was performed by the log rank test.
Results
A total of 182 cases used full median sternotomy, in eight cases combined with a muscle-sparing thoracotomy. The total of minimally invasive procedure successfully performed was 43 divided as follows: triportal unilateral VATS (n=16), bilateral VATS (n=8), combined cervical VATS (n=5), combined subxiphoid VATS (n=7) and finally uniportal VATS subxiphoid (n=7). Another 9 cases initially approached through VATS were converted to sternotomy to allow oncological completeness of resection (n=6), and great vessels invasion unpredictable at preoperative CT examination (n=3). Furthermore, in 4 instances minimally invasive procedures were successfully carried out under non intubated modality through a laryngeal mask and intravenous sedation.
A total of 189 (84.0%) patients had complete resection of the thymoma together with extended thymectomy (Table 3), whereas the remaining 36 patients, had incomplete thymic resections due to the extensive local diffusion of the disease despite induction therapy (n=16) or presence of unexpected metastases (n=8) or evidence of microscopic tumor infiltration on the resection margins (n=12). Complete resection was correlated to the stage of the disease with a 100% resection rate at stage I, 90% at stage II, 45% at stage III and 17% at stage IV, respectively. Symptoms at onset are summarized in Table 1. Notably, asymptomatic patients were 96 (44.6%) whereas venous obstruction was described in 21 patients (9.7%) (Figure 6). MG was diagnosed preoperatively in 80 (37.2%) instances and details of these patients are summarized in Table 4. Other associated pathologies were pure red cell aplasia (n=7), polymyositis (n=5), hypogammaglobulin (n=3), erythroderma (n=1) and limbic encephalitis (n=1).
Table 3
| Variables | Total (n=225) | Complete (n=189) | Incomplete (n=36) | P |
|---|---|---|---|---|
| Age (years) | 47.5±15.3 | 43.4±13.6 | 51.5±17.0 | 0.09 |
| Gender (M:F) | 121:94 (56%:44%) | 104:85 (55%:45%) | 21:15 (59%:41%) | 0.45 |
| Myasthenia gravis, n (%) | 80 (35.6) | 69 (36.5) | 11 (30.6) | 0.63 |
| Masaoka stage, n (% of total) | 0.0001 | |||
| I | 92 | 92 (100.0) | – | |
| II | 76 | 70 (92.1) | 6 (7.9) | |
| III | 41 | 23 (56.1) | 18 (43.9) | |
| IV | 16 | 4 (25.0) | 12 (75.0) | |
| WHO classification, n (%) | 0.001 | |||
| A | 31 (13.7) | 31 | – | |
| AB | 76 (33.8) | 70 | 6 | |
| B1 | 67 (29.8) | 57 | 10 | |
| B2 | 33 (14.7) | 23 | 10 | |
| B3 | 18 (8.0) | 8 | 10 | |
| Diameter (cm) | 5.51±3.25 | 4.37±3.18 | 7.75±5.23 | 0.03 |
| Surgical approach | 0.38 | |||
| Open | 182 (80.9) | 153 | 29 | |
| minimally invasive | 43 (19.1) | 36 | 7 |
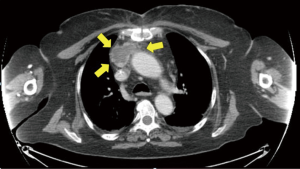
Table 4
| Variables | Total (n=80) | Open (n=68) | Minimally (n=12) | P |
|---|---|---|---|---|
| Frequency (% of total) | 37.2 | 39.5 | 27.9 | 0.24 |
| Age (yrs) | 46.6±14.9 | 44.1±13.7 | 53.1±18.2 | 0.19 |
| Sex (M |
53 |
45 |
8 |
0.11 |
| Class (I–II |
47 |
39 |
8 |
0.76 |
| Stage (I |
35 |
28 |
7 |
0.25 |
| WHO (A, AB, B1 |
60 |
51 |
9 |
0.65 |
| MG score | 19.8±10.3 | 23.5±11.7 | 16.3±8.7 | 0.13 |
| Major morbidity | 9 (11.3) | 8 (11.8) | 1 (8.3) | 0.73 |
| Recurrence, n (%) | 23 (28.8) | 20 (29.4) | 3 (25.0) | 0.75 |
Microscopic thymomas
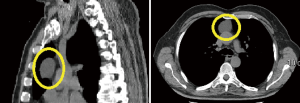
During the examination of thymic specimens removed by sternotomy (n=3) and VATS (n=3) for MG we incidentally encountered 6 microscopic, less than 1 mm in diameter, thymomas (28) and another one was discovered in the wall of a thymic cysts removed by VATS (Figure 7).
Operative and postoperative data
Operative data regarding extent of the resection, blood loss, diameter and histology of the tumor, operative time, stage of the disease are reported in Table 2 and divided for surgical approach. Significant differences were found for blood loss in favor of minimally invasive approaches. Combined resections were performed in 53 instances including pericardium (n=39), lung (n=31), pleural (n=27), diaphragm (n=4), innominate vein (n=21), superior vena cava limited partial resection of the anterior wall with a patch of autologous pericardium (n=3) and entire superior vena cava with prosthetic graft bypass (n=6) (Figure 1) or lateral pericardial patch (n=6) (Figure 2) and entailing more than one structure resected in 41 instances. Eight patients experienced a left (n=2) and right (n=6) phrenic nerve lesion due intraoperative surgical maneuvers (n=4) and tumor involvement (n=4).
Early postoperative data including drainage time, hospital stay, mortality rate and major morbidity rate are summarized in Tables 1 and 2. As expected data concerning minor impact of minimally invasive procedures are significantly evident, whereas 30-day major morbidity rate did not reach the required threshold.
Recurrence pattern
Fifty-five (24.4%) patients recurred (Table 5). Eighteen patients had a local recurrence and 33 distant intrathoracic relapse: lung (n=5), diaphragm (n=2), parietal pleura (n=19) and combined (n=7). In 4 patients we described distant extrathoracic metastases. The surgical approach was not correlated to recurrence pattern (Table 6). Mean disease–free interval for recurrent patients was 30.5±22.3 months. Mediastinal recurrences developed earlier than nonmediastinal ones (mean interval 27±13 vs. 37±25 months; P=0.02). Recurrence rate ranged from 2.1% (n=2) at stage I, to 23.6% (n=18) at stage II, 56% (n=23) at stage III and 75% (n=12) at stage IV, respectively. The recurrences were asymptomatic in 19 patients and the diagnosis was revealed by CT scan abnormalities (Figure 8). Chest symptoms were present in 11 patients, and MG worsen or recurrence was described in 6.
Table 5
| Variables | Total (n=55) | Stage I (n=2) | Stage II (n=18) | Stage III (n=23) | Stage IV (n=12) | P |
|---|---|---|---|---|---|---|
| Frequency (% of stage) | 24.4 | 2.1 | 23.6 | 56.0 | 75.0 | 0.001 |
| Mean time to recurrence (mos) | 30.5±22.3 | 92.5±24.7 | 47.5±43.7 | 22.9±14.7 | 10.5±4.1 | 0.002 |
| Myasthenia gravis, n (%) | 20 (36.4) | 2 (100.0) | 8 (44.4) | 8 (34.8) | 2 (16.7) | 0.17 |
| WHO classification | 0.98 | |||||
| A, n [% of histotype (N=31)] | 3 (9.6) | – | 1 | 2 | – | |
| AB, n [% of histotype (N=76)] | 12 (15.8) | 1 | 4 | 7 | – | |
| B1, n [% of histotype (N=67)] | 13 (19.4) | 1 | 5 | 5 | 2 | |
| B2, n [% of histotype (N=33)] | 14 (42.4) | – | 4 | 5 | 5 | |
| B3, n [% of histotype (N=18)] | 13 (72.2) | – | 4 | 4 | 5 | |
| Previous surgical approach | 0.07 | |||||
| Open, n (% of 182) | 49 (26.9) | 2 | 16 | 22 | 9 | |
| Minimally invasive, n (% of 43) | 6 (14.0) | – | 2 | 1 | 3 | |
| Site, n (%) | 0.91 | |||||
| Original tumor bed | 18 (32.7) | 1 | 9 | 7 | 1 | |
| Pleura | 19 (34.5) | 1 | 5 | 7 | 6 | |
| Lung | 5 (9.1) | – | 1 | 3 | 1 | |
| Diaphragm | 2 (3.6) | – | – | 1 | 1 | |
| Combined | 7 (12.7) | – | 3 | 3 | 1 | |
| Distant metastasis | 4 (7.3) | – | – | 2 | 2 |
Table 6
| Variables | Total (n=71)* | Open (n=39) | Minimally (n=32) | P |
|---|---|---|---|---|
| Previous surgery | ||||
| Sternotomy | 39 | 28 | 11 | 0.5 |
| Minimally invasive | 32 | 13 | 19 | |
| Induction chemotherapy | 49 | 37 | 12 | 0.002 |
| Operation time (min) | 189.4±57.9 | 211.7±64.8 | 175.3±42.9 | 0.55 |
| Intraoperative blood loss (mL) | 252.2±76.1 | 300.9±87.5 | 166.1±55.4 | 0.02 |
| Postoperative drainage (days) | 3.8 ±1.7 | 4.2 ±3.1 | 3.7 ±1.5 | 0.34 |
| Postoperative stay (days) | 5.4 ±2.6 | 5.9 ±4.2 | 5.5 ±2.7 | 0.13 |
| 30-day mortality | 1 | 1 | – | 0.6 |
| Major morbidity, n (%) | 22 (31.0) | 15 (38.5) | 7 (21.9) | 0.03 |
| Completeness redo, n (%) | 53 (74.6) | 34 (87.2) | 19 (59.4) | 0.07 |
| Second recurrence, n (%) | 19 (26.8) | 13 (33.3) | 6 (18.8) | 0.43 |
| Mean second DFI (mos) | 32.6±12.3 | 34.2±13.7 | 25.3±15.9 | 0.25 |
| Second induction chemotherapy | 13 | 9 | 4 | |
| Second procedure for recurrence, n (%) | 19 (26.8) | 14 (35.9) | 5 (15.6) | 0.12 |
| Open | 12 | 10 | 4 | |
| Minimally invasive | 7 | 4 | 1 | |
| Radical second resection, [n (% of 2nd proc)] | 8 (50.0) | 7 (50.0) | 1 (20.0) | 0.31 |
*, also from different center (51 from our series)
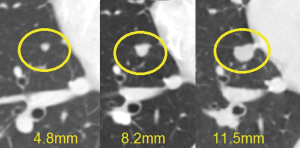
Recurrence treatment and redo surgery
We reported 71 operations for intrathoracic recurrence whose features are summarized in Table 6. Fifty-one patients came from our personal series whilst the remaining 20 ones had previous thymectomy in other centers. Thus, 4 patients refused the second procedure or chose a different center for the treatment of the recurrence. In 32 of the recurred patients we demonstrated a change into a more aggressive WHO histology. Given the extent of the recurrence, 13 patients received preoperative chemotherapy with the same protocol used after the primitive one. The new resection was performed through total median sternotomy (n=11), lateral thoracotomy (n=19), clamshell bithoracotomy (n=5), bilateral staged thoracotomies (n=4), bilateral VATS (n=21) and combined subxiphoid VATS approach (n=11) (Figure 9). Global postoperative major morbidity rate was 31% with a significant lower morbidity for minimally invasive reoperations (P=0.03). Complete resection of the recurrence was achieved in 23 mediastinal and 30 nonmediastinal recurrences (lung, n=7; diaphragm, n=3; parietal pleura, n=21 and combined, n=9). Chemotherapy was usually performed after complete re-resection as well. The remaining 18 patients (7 mediastinal and 11 nonmediastinal) underwent incomplete resection followed by radiotherapy and chemotherapy. We also experienced in 19 (27%) of the 71 operated patients a second recurrence, which deserved a non-surgical medical treatment in 7 and a third operation in 9 instances preceded by new induction chemotherapy cycle in 4 cases. The remaining patients refused any modality of treatment.
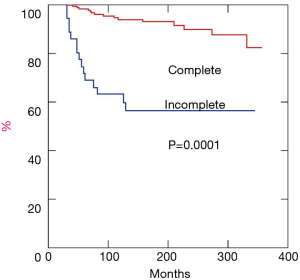
Long-term survivals
Long term results are summarized in Table 7. Patients were followed for a mean period of 174.8±91.1 (range, 27–355) months allowing at least two years of minimum follow up. The longest overall survival was noted by far in patients who achieved complete surgical resection (Figure 10). Once considered only patients who received complete resection overall survival at 5, 10 and 15 years were 98%, 90% and 84%, respectively. In our study group we documented a total of 61 deceased subjects, 30 for thymoma-related causes and the remaining ones not apparently due to this condition, but to cerebrovascular disease (n=11), cardiac disease (n=13), trauma (n=4) and other neoplasms (n=3).
Table 7
| Variables | Total (n=225) | Open (n=182) | Minimally (n=43) | P |
|---|---|---|---|---|
| Follow up (months) | 174.8±91.1 | 197.1±87.8 | 85.5±25.4 | 0.0001 |
| Postoperative MG, n (%) | 8 (3.6) | 8 (4.4) | – | 0.12 |
| Quality of life (PCS) at 5 years | 67.1±14.9 | 59±13.2 | 73±11.1 | 0.03 |
| Local recurrence, n (%) | 18 (8.0) | 14 (7.7) | 4 (9.3) | 0.07 |
| Distant intrathoracic relapse, n (%) | 33 (14.7) | 27 (14.8) | 6 (14.0) | 0.40 |
| Distant metastases, n (%) | 4 (1.8) | 4 (2.3) | – | 0.09 |
| Disease related mortality, n (%) | 30 (13.3) | 26 (14.3) | 4 (9.3) | 0.38 |
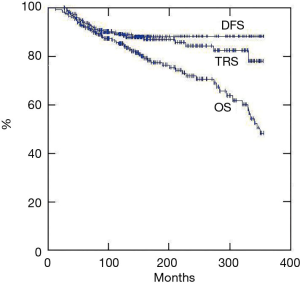
Disease free survival (Figure 11) was estimated at 5 years 91%, at 10 years 82% and at 15 years 78%, respectively. First recurrence pattern is described in the previous paragraph. The stage according to Masaoka proved a significant indicator of overall (P=0.007) and disease free (P=0.004) survivals (Figure 12). Namely, the stage I presented 5-year overall and disease-free survivals of 100% and 95%, stage II of 97% and 92% and stage III of 91% and 87%, respectively. As far as 10-year overall and disease-free survivals were 95% and 91% at stage I, 88% and 85% at stage II, 82% and 79% at stage III. Stage IV was by far the stage with lowest overall survival rates presenting 13% at 5 and 0% at 10 year, respectively.
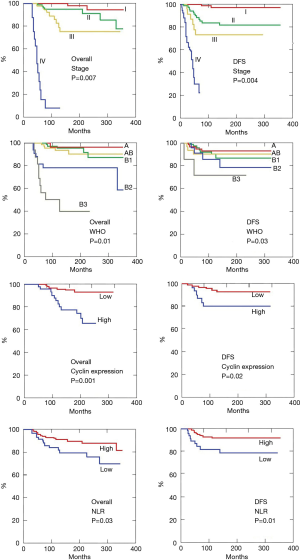
Histology according to WHO was another significant predictor of overall (P=0.01) (Figure 12) and disease-free survival (P=0.03) (Figure 12) and a worse prognosis was experienced in B3 thymomas.
The surgical approach (open vs. minimal) did not influence overall and disease survivals although is important to underline that the observation period for minimally invasive group was significantly shorter than that for open access. Interestingly, we experienced a significantly better quality of life at 5 years after minimally invasive procedures.
The presence of MG did not represent by itself a bad prognosticator. As far as the neurological prognosis of the disease these patients achieved complete stable remission in 65% of the cases at 5 years from the procedure, which is a similar rate obtained in those myasthenic individuals without thymoma. Notably, we documented the onset of MG in 8 patients after a median period of 33 months from thymectomy (29). In this specific setting, the only significant predictor of post thymectomy MG onset was the high preoperative serum acetylcholine-receptor antibodies titer (>0.3 nmol/L).
Biological prognostic factors
The role of biological factors in thymoma has attracted our attention since a long time. Since 2005 we reported the role of cell cycle proteins p53 high, p21 low and p27 low expressions by immunohistochemical staining in the prognosis of thymomas (30,31). So far, we have the chance of reviewing cell cycle expression in 145 thymoma specimens. We found that combination p53 high, p21 low and p27 low was present in 44 patients (30%) and this subset had the lower significant disease-free and overall survivals (Figure 12). At the same time, we documented a worsening of these proteins expression in the recurrence tissue compared to the native ones in 14 of 33 recurred patients: these patients presented by far the poorest surgical results belonging all of them to the incomplete re-resection group and the lowest prognostic subsets.
In the present series we also investigated the role of the ratio of preoperative values neutrophil/lymphocytes, which is a never tested prognostic marker in the case of thymoma. These values are easily retrievable from the database and proved a reliable index of both tumor aggressiveness and host immune-competence. We found that patients with a preoperative ratio greater than the median value disclosed shorter disease-free and overall survivals (Figure 12).
Discussion
Herein we exposed a retrospective study concerning thymectomy for thymomas spanning three decades. In this period extended thymectomy using the different approaches was performed by the same surgeon (TCM) through full median sternotomy, multiportal VATS and more recently subxiphoid VATS approaches. Data emerging from our surgical experience matches with those expressed in the literature dedicated to this tumor (32). However, it is important to note that during the span of time analyzed, surgical care as well as surgical approaches and anesthetic management had considerably changed and improved leading to better results.
Thymoma remains the most common thymic tumor. In general, they have been considered as benign lesion because of their very indolent grow pattern. However, local invasion, pleural dissemination as well as lung and diaphragmatic involvement and even recurrence and systemic metastases are all signs of relatively low malignant potential. Thus, these rare tumors should be faced as malignancies (33).
Our experience hinges fundamentally on the full sternotomy. This access always satisfied our aim of accomplishing extensive and complete resections as much as possible that remains one of the most important determinant of long-term survival. The mise a plat of the mediastinum between the two hila and from the neck to the diaphragm represents the cornerstone to prevent recurrences in the thymic bed, and in the pleural cavities and in the lungs as well. Sternotomy has been a standard mode of resection for thymoma both in presence or absence of MG. Furthermore, full sternotomy allows us the actions, whenever required, finalized at removing concomitant lesions in the pleural surfaces, lungs and diaphragm. This approach also permits the extended procedures in the case of great vessels and pericardium involvement. There is no doubt that sternotomy offers better visualization and wider operative field. In the lack of alternative options these advantages forced the surgeon to use this approach despite high and prolonged morbidity and long-term mortality. Poststernotomy complications increases with obesity, diabetes, advanced age and smoking habit. Steroid chronic treatment further increases the risk of wound infection and sternum dehiscence. As consequence, sternotomy may hardly compromise the normal daily activities for a long time. In addition, rate of complications and recurrences reported in retrospective and non-randomized studies does not consent to clearly conclude that sternotomy is a better approach than VATS for treatment of thymomas. Notwithstanding sternotomy remains still irreplaceable in locally advanced tumors that need wide demolitions and elaborate reconstructions. For these reasons we still recommend that sternotomy should belong to the background of any surgeon before approaching novel minimally invasive techniques.
Our experience with VATS is more recent and numerically smaller than that acquired with sternotomy. However, we experienced all the advantages of this approach, which is gradually replacing full sternotomy unless in patients with evident invasive neoplasms. In this setting, as other surgeons, we paid a long time to acquire a better anatomic knowledge from the view point of the lateral side chest approach. Similarly, we spent a consistent period in learning to work in a more limited space that makes quite much hard dissecting the neoplastic thymic gland from the important adjacent structures.
On these premises, in 1995 we started with the left VATS extended thymectomy for non-thymomatous MG (18,23). Subsequently, after having gained a significant experience, in 2005, we elected to perform thymectomy using VATS for small and early-stage thymomas as well. This innovative approach progressively replaced sternotomy providing proper visualization and enabling complete extended resection. At start, we have preferred the left approach for anatomical reasons that favor a more extended and safe thymic resection (18). However, both sides can be successfully employed and the choice of the side is in general related to the surgeon’s preference although the anatomical prevalence of one side over the other observed at CT is the hinge criteria (2,20,33-35). A number of technical modifications have been proposed over the years and to date VATS thymectomy has progressively affirmed all over the world as surgical option (36,37).
The real efficacy, safety and long-term oncological outcomes of VATS are still under debate of thoracic surgeons (38). Results remain controversial when comparing the thoracoscopic and transsternal thymectomy for the treatment of thymoma.
VATS proved more advantageous in the immediate postoperative period and short-term outcomes as well. Furthermore, VATS approach compared with classic sternotomy entails less intraoperative blood loss, negligible wound complications, as well as less postoperative acute and chronic pain, better cosmetic sequelae, reduced risk of respiratory and cardiac related complications. In general, patients receive benefits from lower hospital stay and rapid return to normal activities originating from VATS operation. Thus, we noticed a faster acceptance of the operation among both patients and physicians.
Given these clear advantages and minor complications compared to sternotomy, VATS has presently gained a wide popularity and superimposed to open accesses in the treatment of Masaoka stage I, II and part of III thymomas (33,39). At these stages equivalent oncological outcomes have been observed for VATS thymectomy when compared with transsternal thymectomy (35,40). Obviously, the well documented invasion of the innominate vein axis as well as of great vessels do not enter in the criteria for thoracoscopic treatment and, generally speaking, Masaoka stage III and IV thymomas remains among the group that takes advantages from sternotomy. There are published data that demonstrated comparable mortality results in thymectomy by total sternotomy and VATS (36).
A major concern deserves the recurrence after VATS because of manipulation effect of the endoscopic instruments. This can happen especially on the largest tumors that may facilitate pleural dissemination. Kimura et al. (41) reported that 3 out of 45 patients undergoing thoracoscopic operation for thymoma greater than 5 cm recurred with pleural dissemination and namely 2 of those had capsule injuries during maneuvers. Conversely, pleural recurrence after thoracoscopic thymectomy resulted comparable to those after sternotomy (42). The employ of the endobag is mandatory for taking out bulky tumors and any fragment of fat mediastinal resected tissue time-by-time in order to reduce the risk of seeding. To date there are not definitive guidelines regarding the most appropriate size of thymoma considerable an indicator for thoracoscopic removal. There is a quite unanimous agreement in indicating that thoracoscopic thymectomy is technically safe and feasible for thymomas that do not exceed 5–6 cm in maximum diameter. However, VATS removal of larger thymomas may predispose to recurrence (43). In these cases, it can be easier to extract create from a subxiphoid utility.
Despite these controversies VATS has progressively conquered more consensus particularly among young surgeons. It is clear that this approach leads to early operations, especially in those patients affected with concomitant MG thus enabling the physicians to recommend a surgical treatment with minimal chest pain, less trauma and better quality of life (44).
There are significant variations in VATS approaches for thymoma. The use of tridimensional operating imaging and camera has also revolutionized the VATS technique. In recent years use of robotic technology in thymic surgery became popular. Robotic thymectomy are now performed in dedicated centers all around the world. Results show that robotic thymectomy in selected thymomas can achieve oncologic outcome equivalent to the open and thoracoscopic approaches (45-49).
In spite of variety of VATS approach our preference currently remains for a left-sided three or uni-portal VATS approach that provides the best exposure for stage I and II thymomas and in selected stage III thymomas, particularly in those patients affected with MG. Having gained experience we can sufficiently dominate the innominate axis, venous junction, aorto-pulmonary window and both phrenic nerves as well as cardio-phrenic angles. In general invasive thymomas are not resected by VATS because of technical difficulties and possible oncological compromise for tumor seeding (50). In this group stage III thymomas are included and the preoperative studies can be useful to exclude a VATS approach. However, improvements in VATS technique as well as gained experience allow safe resection of selected invasive tumors avoiding sternotomy.
In recent years a subxiphoid VATS approach has been introduced as another alternative route to sternotomy for patients suffering of Masaoka’s stage I to III thymomas (51-56). It allows surgeon to see both phrenic nerves and reach high into anterior mediastinum. Chest CT can help surgeons to verify the feasibility and safety of subxiphoid thymectomy. Obviously, this approach appears as more advantageous because of the relatively painless, avoidance of the risk of infection or disruption of sternal wound, wider visualization and cephalad dissection up to cervical and thyroid area. These features enable complete resection of the thymus and the fat tissue yet conserving satisfying and wide access to the chest and total respect of cosmesis. In invasive thymoma this route is not indicated due to limited visualization and dissection. Our experience with this approach is still numerically inadequate for a statistically significant evaluation. However, we appreciated its advantages in nonthymomatous patients and in reoperations for mediastinal limited recurrent thymomas. Before facing these difficult operations, we recommend a progressive stepwise path including first VATS thymectomy for nonthymomatous patients and thereafter progressing towards small and well-capsulated thymomas and reaching to larger and more advanced tumors.
Masaoka stage I thymoma is the most frequent tumor in our experience. All patients underwent a complete simple en-bloc resection of the gland including the tumor achieving an overall crude survival of 98% at 5 years and 93% at 10 years, respectively. No patient at this stage received adjuvant therapy. Nowadays, at this stage we give our preference to VATS approach either through chest or subxiphoid uniportal approach. VATS was safe and feasible in this stage and 5-year overall and disease-free survivals were comparable to those achieved with the traditional open approach. We reported only two patients with recurrences. They were both small recurrence and sited in the thymic bed and they were removed by minimally invasive approach. In all 35 MG patients operated at this stage the myasthenic symptoms were the earliest and often the unique manifestation of the neoplasm. All recurrences were in myasthenic patients at stage I after a mean period of 22 months. Re-onset or worsening of myasthenic symptoms revealed the occurrence of the recurrence. All these patients are alive and in complete neurological remission after 10 years from the second operation.
Resectability rate is very high stage I, but it progressively decreases with the increment of the stage as well as with the surgeon’s skill to manage the invasion of the neighboring structures. In encapsulated thymoma especially in young and myasthenic patients, surgical resection should avoid capsule breaching in order to decrease the possibility of neoplastic seeding.
For this stage no adjuvant treatment is recommended because the risk of recurrence is very limited after complete resection. In those patients who cannot tolerate a surgical procedure for medical reasons irradiation does not influence overall survival.
One third of our patients were assigned to the Masaoka stage II, which in general represents 25% of the entire amount of thymomas (33,57). In the majority of the cases surgery results in complete resection ranging from 40% to 100% (32,58). We reported in this stage a 92% of resectability. Due the frequent adherence to the mediastinal pleura they tend to recur locally and even to metastatize despite adjuvant radiotherapy (59). This implies different opinions in the postoperative strategy. WHO B2 and B3 types stage II tumors demonstrate a high incidence of recurrence thus needing systemic adjuvant treatment (60,61). This stage implies heterogeneous conditions that originates a variety of postoperative modalities of treatment. In this setting we found supporters of radiation therapy for all patients or of radiation only in large tumors (greater than 5 cm in diameter) (62,63). Others suggests radiotherapy in cases of radiographic evident invasiveness or for WHO type B (64,65). Others more advocate no radiation at all (66). These different opinions are based on the finding that in retrospective analysis adjuvant treatment does not offer a clear benefit over the surgery alone (67). These patients were found to present more frequent pleural recurrence that is outside from the mediastinal irradiation field (62,68). This fact may suggest the supposed lack of efficacy of postoperative radiation therapy. On these findings adjuvant chemo-radiotherapy was accomplished for tumor greater than 5 cm and in WHO B histotype (60). This modality significantly reduced recurrences and relapses. We used adjuvant radio and chemotherapy in case of young subjects (less than 50-year-old) with larger tumors and/or positive resection margins whatever the WHO histologic subtype. No recurrence was observed in this setting whereas six pleural recurrences were observed after sole adjuvant radiotherapy. All the recurrences at this stage were surgically treated.
Twenty-eight patients at this stage presented MG, that was the first symptom of the thymoma. Eight patients recurred within a mean period of 32 months either in mediastinum or pleura. All were resected with an improvement of myasthenic symptoms.
Forty-one (18.2%) of our thymomas were at Masaoka stage III, which usually represents one quarter of all tumors (33). This stage entails different conditions of invasiveness to the neighbor structures such as pericardium, visceral pleura, great vessels and phrenic nerves. When possible, en-bloc resection remains the mainstay in this stage.
Approximately one half of stage III patients can be completely resected with a wide range of resectability from 0% to 89% (2). In our series 23 out of 41 patients had complete resection en-bloc of the tumor and gland with a resectability rate of 56%. A vast majority of the patients were approached through sternotomy; indeed initially we did not operate stage III by VATS and we started only in a second time and in selected patients. Thirty patients received induction chemotherapy because of imaging evidence of invasion neighboring structures in particular vascular. Nobody received preoperative radiation therapy.
Association with MG was found in 15 patients at this stage and in 11 resections was complete. Nine of them had received induction chemotherapy that was well tolerated. All patients developed partial remission of myasthenic symptoms. After resection 8 patients recurred.
After resection vena cava can reconstructed (69,70) by a conduit of expanded polytetrafluoroethylene (PTFE) (71) or by bovine pericardium (72). A patch of autologous pericardium or other material can be used in limited partial resection of the anterior wall of the superior vena cava. Phrenic nerve is often involved and it can be unilaterally resected. In myasthenic patients a diaphragmatic plication can be added in order to preserve respiratory function.
Cumulative 10 years overall survival at 10 years was 75%. We experienced 23 recurrences that is 56% recurrence rate. Most common site are the pleura and the tumor bed. This finding should endorse the use of postoperative radiotherapy. All patients at this stage received routine postoperative radiation therapy independently from sex, age, WHO type histology, tumor size, grade and previous induction chemotherapy. However, 7 elderly patients refused radiotherapy because of comorbidity, 6 of those recurred and 4 were operated upon.
The impact of adjuvant radiotherapy in patients with thymoma remains unclear. Postoperative radiation therapy is recommended by some investigators (73). No local recurrence was observed by Urgesi in 33 patients completely resected with postoperative radiotherapy (74). Curran et al. observed mediastinal recurrence rate of 53% at stage II and III in patients non-treated postoperatively compared to no recurrence in those undergoing adjuvant radiotherapy (75). Similarly Monden et al. (76) in patients at stage III and IV presented a 20% rate of recurrence after adjuvant radiotherapy which is better than 50% observed in patients without adjuvant irradiation.
In incomplete resected patients adjuvant radiation can decrease the recurrence rate among patients with residual disease. Curran et al. observed no mediastinal recurrence after radiotherapy for incomplete resection (75). Similar results have been achieved by some authors (74,77) reporting low rates of mediastinal recurrence in patients with gross residual tumors treated with adjuvant irradiation. Conversely, Mangi et al. (78) and Kondo & Monden (40) observed that recurrence rate was similar in patients treated with surgery alone and those followed by radiation.
These contradictory opinions suggest that all stage II and stage III patients should have a strict follow up by chest CT at timed-intervals.
Fifteen patients presented clinical MG, 10 patients of those underwent neoadjuvant chemotherapy. Chemotherapy was well tolerated. Eight patients recurred in mediastinum (n=3) or pleura (n=5) after a mean disease-free interval of 21 months. Four of those had undergone neoadjuvant chemotherapy. All of them were reoperated upon.
Masaoka stage IV thymomas represent the most advanced form of this thymic tumor. Surgery alone cannot reach complete resection given surgery alone is often not radical and does not provide satisfactory outcomes. There are few studies reporting the management of Masaoka stage IV thymoma. In general, they include both stage IVA and IVB and span many decades. This stage manifests a disseminated disease where complete resection can be hardly achieved unless sporadic cases. However, in recent years pleurectomy and extrapleural pneumonectomy have been successfully carried out (79). Similarly to others, in patients with pleural and pericardial dissemination and free of distant metastases we have experienced acceptable outcomes using multimodality treatment (80). Also at this stage the purpose of multimodality therapy is to reduce tumor volume allowing subsequent surgical resection or adjuvant radiotherapy (80).
Our analysis has shown that 4 patients after induction chemotherapy and were widely resected. The two cases had MG and they both recurred despite complete resection. Adjuvant chemotherapy for stage III and IVA increases the resectability rate to 72% that is as compared to surgery alone ranging from 50–25% (2).
Thymomas are associated with a numerous concomitant pathologic condition generally called parathymic syndromes. The most frequent of these is MG, which occurs in approximately 45–65% of the patients with thymoma (2). The histologic pattern more frequently associated with MG are the cortical subtype or B types according to the WHO classification. Conversely, 10% to 25% of myasthenic patients are found to have thymoma. MG is recognized to be an autoimmune disease that attacks neuromuscular junction characterized by neuromuscular weakness and fatigability. MG is caused in 85% of cases by AChR antibodies that are the main causes of muscular weakness in thymoma MG (81). In our series thymoma MG is equally distributed in males and females and occurs at any age with a peak onset around 50 years. Thymoma MG tends to be more severe than early onset non-thymoma MG. The occurrence of a thymoma per se does not give a more severe MG (82). For decades MG was deemed to have a negative impact on thymoma prognosis. On the other hand, some investigations have recently showed a positive impact on survival probably due to steroid use, better treatment of concomitant medical problem and earlier detection of the neoplasm given the stricter follow up (83,84). Until now it is unclear how a thymoma cause MG or viceversa. Over time no difference in MG severity and evolution has been showed in thymomas and non-thymoma patients as well. Both groups of patients improve in the same degree after pharmacological management and thymectomy. We found no difference in modality of thymectomy both through sternotomy and VATS. Equally a thymoma completely removed does not mean worse MG prognosis. The thymomas MG patients and age-matched nonthymoma MG patients share similar MG long term prognosis and results. In MG patients the complete thymoma excision does in most cases cure the neoplasm but patients will continue to present myasthenic symptoms despite the procedure. This occurrence entails the endurance of pharmacological therapy and the need of a close follow up. Neoadjuvant induction chemotherapy for myasthenic patients at stage II–IV is in general well tolerated and effective also for neurologic symptoms (59,85).
As for non thymomatous MG benefits are not immediate but they progress in a long period that can last up to 15 years and more. Myasthenia can occur even after several years from complete thymectomy for non-myasthenic thymomas depicting the scenario of “post-thymectomy MG”. (86,87). We have recently investigated this peculiar association in our series and we found that the only factor associated with postoperative MG onset was the high preoperative level of antibodies against the receptor for acetylcholine (AChR-Ab) (29). In this setting the production of AChR-Ab is primarily sited in the germinal centers of residual or ectopic thymic tissue persisting in the mediastinal region despite most aggressive ablation of thymic tissue (88,89). We hypothesized that the thymoma might have an inhibitory mechanism on thymic tissue foci. This tissue can be activated after removal of the neoplasm, with the production of AChR-Ab from their germinal centers.
Among the parathymic syndromes the pure red cell aplasia and hypogammaglobulin are the most common associated diseases both occurring in 2% to 5% of the patients (90). Half of the patients with pure red cell aplasia are found to have a thymoma (91). Conversely, only 10% of the patients with hypogammaglobulin have a thymoma. To date, the mechanism of these associations remains unclear. Pure red cell aplasia is a poor prognostic factor. It is accompanied by a higher morbidity and a shorter disease-free interval (92) In our series mortality rate of these patients was significant lower and namely the overall survival was 71% at 5 years.
In 1976, Rosai & Levine (93) introduced the term “microscopic thymoma” to define lesions histologically characterized by epithelial proliferation, smaller than 1mm in diameter, usually multifocal, sited in the cortex or medulla, in absence of a macroscopically evident thymic tumor. These lesions are observed in the resected specimen patients undergoing thymectomy suffering from MG (94). After having reviewed our series we found microscopic thymomas in 6 myasthenic patients and the features of these patients do not differ from those already reported (95). The last case was discovered after resection of a thymic cyst and this condition is already described in the literature (96).
All the topics entailing thymoma recurrences as well as many other topics concerning thymic tumors have been deeply analyzed by Detterbeck and Parsons (2). The present retrospective analysis noticed the presence of 55 patients who recurred after a mean time of 30 months from thymectomy. This value coincides with many reports from the literature. The specific behavior of the recurrence shows the relatively potential malignancy of these tumors. Indeed, in opposition to other thoracic malignancies, these recurrences do not imply a poorer prognosis or shorter survival time given the indolent behavior of thymoma and their slow growth. Thymomas can recur at any stage, at any time and independently from the surgical procedure either complete or incomplete. The average recurrence rates are 3% to 5% for Masaoka stage I thymomas and progressively increases to 16% for stage II and 26% for stage III (64,97-99). Stage IV tumors recur with a wide variability ranging from 25% to 80% (64,97-99). Because of low growth of these tumors the average time to recurrence is reported around 5 years ranging from 3 to 7 years (64,83,85,97). However, recurrences have been incidentally described after 3 months and even after 15 years (64,83,97). Local recurrences are more frequently observed than the distant ones. One half of the local ones appears as nodules sited in parietal pleura or in the lung. The other local recurrences are located in the mediastinum mainly growing as isolated but sometime also as scattered nodules on the pericardium surface. In our series recurrence are equally distributed in the pleura and in the mediastinum. Distant metastases are mostly located in liver and bones. We observed four patients with liver metastases after a mean period of 163 months.
In general redo surgery is performed in patients with limited recurrence and good general conditions that are followed in the majority of the instances a good result. The optimal strategies for treating recurrent thymomas remains still now controversial (2). Good results in term of survival have been reported after resection (64,98,100,101). Other authors advocate chemotherapy as a treatment of choice (102). An aggressive behavior had been always proposed given the slow growth and the relative facility of radical resection. Completeness of resection at the time of redo surgery for recurrent thymoma is the most important determinant of long-term survival (98,103). Approximately 50–75% patients with recurrence are operable (64,74,98,104,105) and complete resection is feasible in 45–70% of the instances with a significant long-term survival 70% at 5 years and 50–60% at 10 years, respectively (63,74,98). On the contrary, in patients with incomplete resection of the recurrence survival is significantly decreased to 0–25% at 5 years and to 0–10% at 10 years, respectively (2,83,98). There are reported cases of a disease-free interval longer than 13 years after recurrence removal (83,105). In our series we have performed reoperations in a total of 71 patients, 39 after sternotomy, 29 of which were re-approached through thoracotomy (Table 6). More recently, especially in the case of small recurrences in the thymic bed, whatever the approach, we have given our preference to a subxiphoid VATS, which allowed complete resection in all cases.
A second recurrence was observed in only 16–25% of patients after complete resection of the first recurrence (83,105). In the recurrence repeat surgery complete resection remains the first choice of treatment because the recurred tumor is more aggressive and limited to the chest (106). The onset of a second recurrence after a long timespan induces our patients to refuse a third operation. Recurrence rate after VATS thymectomy ranges from 0% to 6.7% and 5-year disease free survival ranges from 83–96% (107).
It is specific merit of our center to have firstly investigated the role of gene expression in the prognosis of thymoma (30,31). Cyclins and namely p27, p21 and p53, are important proteins involved in control of the cell cycle. Level of expression of their genes was able to predict both disease free and long-term survivals in encapsulated (30), radically resected (31) and recurrent (106) thymomas. Furthermore, these markers reveal the aggressiveness of the tumor and can also be indicators of response to neoadjuvant therapy (108). In the present study we extended our sample to 145 cases achieving similar results to the previous investigations. Interestingly, we demonstrated a worsening of cell-cycle expression in those patients who recurred and this was strictly related to the oncologic prognosis of the disease.
Similarly to other neoplastic conditions (109) we tried to correlate prognosis with preoperative ratio neutrophil to lymphocytes. The increment of this ratio can be considered a valid predictor of prognosis, with the increment of neutrophils that may have an inflammatory resulting in pro-cancer activity and decrement of lymphocytes that might weaken immunologic and anticancer activity. Interestingly, we found that an elevated preoperative ratio can influence both disease-free and overall survivals and to our knowledge this one of the first reports in literature investigating this specific topic (110,111).
Conclusions
Our conclusions are not so different from those reported in the literature, especially if deriving from series spanning many decades. These tumors are slow-growing and often asymptomatic neoplasms of the anterior mediastinum. The presence of myasthenic symptoms may favor their diagnosis especially at early stages. Complete resection remains the golden standard of the curative treatment whatever the surgical approach. At surgery every effort need to be made to achieve the most complete resection. Similarly to non-thymomatous thymectomy we would state that also in thymectomy for thymoma the resection should include the whole mediastinal tissue. When necessary removal should be extended to the neighboring involved structures such as pericardium, left brachiocephalic vein, mediastinal pleura and superior vena cava. This large vessel can be successfully reconstructed by artificial conduit or partially with a patch autologous pericardium. When a complete resection of the mass is not feasible even an incomplete resection might offer a somewhat better survival than that reported in non-treated patients, but at this propos opinions and results are discordant. In these conditions adjuvant radio or chemotherapy is alternatively proposed but with incomplete details about outcomes.
Completeness of resection is the most important determinant of long-term survival. Masaoka stage of the tumor as well as WHO histology are consistent independent prognostic factors in the largest series of thymoma. No other classic prognostic factor has been found to be of value. In this regard we demonstrated the prognostic utility of biologic factors such cell cycle expression and in the present study the ratio neutrophil to lymphocytes.
The associated MG no longer appears as a negative prognostic factor and no difference in survival of patients with or without MG was observed. Full median sternotomy has been used for resection of thymoma at any stage until the development of VATS. This minimally invasive technique has gained acceptance as a viable option both thymectomy in non-thymomatous and in thymomatous patients. There are several advantages of VATS over open access including better cosmesis, decreased postoperative pain and similar long-term outcomes. Nowadays, in spite of controversies VATS thymectomy for thymoma is a consolidated surgical procedure. In recent years the use of robotic technology has provided a further option to the surgery of thymomas and robot-assisted thymectomy for thymoma are now performed in many dedicated centers with equal oncologic results to the open and thoracoscopic approaches.
Due to the slow-growth behavior of this tumor, thymoma patients are at risk for late mediastinal or intrathoracic recurrences. Thus, they should carefully be followed for a very long time. In general, local recurrences are easily removed by surgery and more than once. The recurrence of thymoma does not imply a poor prognosis. Thus an aggressive approach is justifiable and effective in the majority of cases. The survival in patients operated for recurrence appears to be similar to that of patients without recurrence.
Multimodality therapy including radio or chemotherapy is used as adjuvant or neoadjuvant treatment in the most locally-advanced thymomas. At this regard there are no specific guidelines and long-term results need to be further analyzed. At this stage adjuvant radiotherapy is the most used treatment whatever the completeness of the resection.
Despite so many bias due to the long time-span and to the scientific and technical advances in this field, our analysis permits the evaluation of the whole aspects of this mediastinal malignancy that have always and will continue to attract the attention of the thoracic surgeons. At the end of this 30-year journey we are happy to show these images that belong now to the history of the medicine (Figure 13). No progress is possible without a long study and great efforts.
Acknowledgments
We feel deeply in debt with Dr. Gabriele Mazzitelli for his precious and irreplaceable work of researching the scientific material indispensable to write this paper. We also acknowledge great merits to the Myasthenia Gravis Unit of the Tor Vergata University, for professionalism and efforts covering a 30-year period and namely Giorgio Bernardi, Roberto Massa (neurologists), Mario Dauri, Eleonora Fabbi (anesthesiologists), Francesca Leonardis, Silvia Natoli (intensivists), Guglielmo Manenti (radiologist), Daniela Brugnoli, Simona Lezzerini, Anna Maria Servadio (physiotherapists), and all nurses in the operative room, intensive care unit and ward.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Mediastinal Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.07.16). The series “Mediastinal Surgery” was commissioned by the editorial office without any funding or sponsorship. TCM served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Tor Vergata Foundation Institutional Review Board authorized the revision of the charts. Due to the long time period considered and the different clinical institutions involved permission was issued with waiver of informed consent from the uncontactable patients (PTV No. 2017-187-3).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WT, Brambilla E, Muller-Hermelink HK, et al. WHO classification of tumors, pathology and genetics of tumors of the lung, pleura, thymus and heart. Lyon, France. IARC press; 2004;
- Detterbeck FC, Parsons AM. Thymic tumors: a review of current diagnosis, classification and treatment. In: Patterson GA, Cooper JD, Deslauriers J, et al, eds. Pearson’s thoracic and esophageal surgery. 3rd ed. Philadelphia, PA: Churchill Livingstone; 2008:1589-614.
- Lardinois D, Weder W. Diagnostic strategies in mediastinal mass. In: Patterson GA, Cooper JD, Deslauriers J, et al, eds. Pearson’s thoracic and esophageal surgery. 3rd ed. Philadelphia, PA: Churchill Livingstone; 2008:1506-20.
- Shimosato Y, Mukai K, Matsuno Y. Tumors of the mediastinum. In: Silverberg SG, editor. AFIP Atlas of tumor pathology, 4th serie: 11, Washington DC: Armed Forces Institute of Pathology, 2010.
- Kalhor N, Moran CA. Thymoma: current concepts. Oncology (Williston Park) 2012;26:975-81. [PubMed]
- Mineo TC, Ambrogi V. Thymomas: a continuing challenge. Oncology (Williston Park) 2012;26:984-7. [PubMed]
- Mineo TC, Ambrogi V. Video-assisted thoracoscopic thymectomy surgery: Tor Vergata experience. Thorac Cardiovasc Surg 2015;63:187-93. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Schillaci O. May positron emission tomography reveal ectopic or active thymus in preoperative evaluation of non-thymomatous myasthenia gravis? J Cardiothorac Surg 2014;9:146. [Crossref] [PubMed]
- Ambrogi V, Mineo TC. Benefits of comprehensive rehabilitation therapy in thymectomy for myasthenia gravis: a propensity score matching analysis. Am J Phys Med Rehabil 2017;96:77-83. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Ricci C, Mineo TC. Chirurgia del timo: correlazioni anatomo-cliniche nei tumori del timo. Atti della Società Italiana di Chirurgia Toracica 1974:152-75.
- Ricci C, Mineo TC, Zaccara G. Chirurgia del timo: risultati della terapia chirurgica nei tumori del timo senza miastenia. Atti della Società Italiana di Chirurgia Toracica 1974:382-94.
- Kittle CF. Which way in? The thoracotomy incision. Ann Thorac Surg 1988;45:234. [Crossref] [PubMed]
- Julian OC, Lopez-Belio M, Dye WS, et al. The median sternal incision in intracardiac surgery with extracorporeal circulation: a general evaluation of its use in heart surgery. Surgery 1957;42:753-61. [PubMed]
- Kondo K. Therapy for thymic epithelial tumors. Gen Thorac Cardiovasc Surg 2014;62:468-74. [Crossref] [PubMed]
- Masaoka A. Extended transsternal thymectomy for myasthenia gravis. Chest Surg Clin N Am 2001;11:369-87. [PubMed]
- Mineo TC, Pompeo E, Ambrogi V, et al. Adjuvant pneumomediastinum in thoracoscopic thymectomy for myasthenia gravis. Ann Thorac Surg 1996;62:1210-2. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Ambrogi V. Video-assisted thoracoscopic thymectomy: from the right or from the left? J Thorac Cardiovasc Surg 1997;114:516-7. [Crossref] [PubMed]
- Mineo TC, Ambrogi V. Moving around the thymus: more technology, more safety, more efficiency. J Thorac Cardiovasc Surg 2016;152:280-1. [Crossref] [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms definitions and policies for minimally invasive resections of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Ambrogi V, Mineo TC. Active ectopic thymus predicts poor outcome after thymectomy in class III myasthenia gravis. J Thorac Cardiovasc Surg 2012;143:601-6. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Ambrogi V, et al. Video-assisted approach for transxiphoid bilateral lung metastasectomy. Ann Thorac Surg 1999;67:1808-10. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Lerut T, et al. Thoracoscopic thymectomy in autoimmune myasthenia: results of the left-sided approach. Ann Thorac Surg 2000;69:1537-41. [Crossref] [PubMed]
- Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol 2011;6:S1691-7. [Crossref] [PubMed]
- Osserman KE. Myasthenia gravis. New York: Grune & Stratton; 1958:80-1.
- Barohn RJ, McIntire D, Herbelin L, et al. Reliability testing of the quantitative myasthenia gravis score. Ann N Y Acad Sci 1998;841:769-72. [Crossref] [PubMed]
- Ambrogi, V, Mineo TC. Role of physiotherapy in myasthenic patients. In: Mineo TC (Ed.) Novel Challenges in Myasthenia Gravis. New York: Nova Science Publishers; 2015:311-8.
- Mineo TC, Ambrogi V. A curious rarity of the thymus gland: the microscopic thymoma. Mediastinum 2018;2:16. [Crossref]
- Mineo TC, Tamburrini A, Schillaci O, et al. Onset and evolution of clinically apparent myasthenia gravis after resection of non-myasthenic thymomas. Semin Thorac Cardiovasc Surg 2018;30:222-7. [Crossref] [PubMed]
- Baldi A, Ambrogi V, Mineo D, et al. Analysis of cell cycle regulator proteins in encapsulated thymomas. Clin Cancer Res 2005;11:5078-83. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Mineo D, Baldi A. Long-term disease-free survival of patients with radically resected thymomas. Cancer 2005;104:2063-71. [Crossref] [PubMed]
- Detterbeck FC, Asamura H, Crowley J, et al. The IASLC/ITMIG thymic malignancies staging project: development of a stage classification for thymic malignancies. J Thorac Oncol 2013;8:1467-73. [Crossref] [PubMed]
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J 2013;126:2186-91. [PubMed]
- Ng CS, Wan IY, Him AP. Video-assisted thoracic surgery thymectomy: the better approach. Ann Thorac Surg 2010;89:S2135-41. [Crossref] [PubMed]
- Odaka M, Tsukamoto Y, Shibasaki T, et al. Surgical and oncological outcomes of thoracoscopic thymectomy for thymomas. J Vis Surg 2017;3:54. [Crossref] [PubMed]
- Pennathur A, Qureshi I, Schuchert MJ, et al. Comparison of surgical techniques for early-stage thymoma: feasibility of minimally invasive thymectomy and comparison with open resection. J Thorac Cardiovasc Surg. 2011;141:694-701. [Crossref] [PubMed]
- Li Y, Wang J. Left-sided approach video-assisted thymectomy for the treatment of thymic disease. World J Surg Oncol 2014;12:398. [Crossref] [PubMed]
- Youssef SJ, Louie BE, Farivar AS, et al. Comparison of open and minimally invasive thymectomies at a single institution. Am J Surg 2010;199:589-93. [Crossref] [PubMed]
- Singhal S, Kaiser LR. Comparison of stage I-II thymoma treated by complete resection with or without radiation. Ann Thorac Surg 2003;76:1635-41. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84. [Crossref] [PubMed]
- Kimura T, Inoue M, Kadota Y, et al. The oncological feasibility and limitations of video-assisted thoracoscopic thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2013;44:e214-8. [Crossref] [PubMed]
- Lucchi M, Mussi A. Surgical treatment of recurrent thymomas. J Thorac Oncol 2010;5:S348-51. [Crossref] [PubMed]
- Tseng YC, Tseng YH, Kao HL, et al. Long term oncological outcome of thymoma and thymic carcinoma - an analysis of 235 cases from a single institution. PLoS One 2017;12:e0179527 [Crossref] [PubMed]
- Bachmann K, Burkhardt D, Schreiter I, et al. Long-term outcome and quality of life after open and thoracoscopic thymectomy for myasthenia gravis: analysis of 131 patients. Surg Endosc 2008;22:2470-7. [Crossref] [PubMed]
- Batirel HF. Minimally invasive technique in thymic surgery: a worldwide perspective. J Vis Surg 2018;4:7. [Crossref] [PubMed]
- Wang H, Gu Z, Ding J, et al. Perioperative outcomes and long-term surgical in clinically early-stage thymic malignancies: video-assisted thoracoscopic thymectomy versus open approaches. J Thorac Dis 2016;8:673-9. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Kaba E, Cosgun T, Ayalp K, et al. Robotic thymectomy: a new approach for thymus. J Vis Surg 2017;3:67. [Crossref] [PubMed]
- Mussi A, Fanucchi O, Davini F, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2012;41:e43-6. [Crossref] [PubMed]
- Agasthian T. Beyond the limits, extreme minimally invasive surgery in invasive thymic tumours. J Vis Surg 2017;3:58. [Crossref] [PubMed]
- Zielinski M, Czajkowski W, Gwozdz P, et al. Resection of thymomas with use of the new minimally-invasive technique of extended thymectomy performed through the subxiphoid-right video-thoracoscopic approach with double elevation of the sternum. Eur J Cardiothorac Surg 2013;44:e113-9. [Crossref] [PubMed]
- Hsu CP. Subxiphoid approach for thoracoscopic thymectomy. Surg End 2012;93:334-6.
- Suda T. Single port thymectomy using a subxiphoid approach: surgical technique. Ann Cardiothorac Surg 2016;5:56-8. [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results. Eur J Cardiothorac Surg 2016;49:i54-8. [PubMed]
- Suda T. Subxiphoid VATS thymectomy for myasthenia gravis. Video-assist Thorac Surg 2017;2:15. [Crossref]
- Yano M, Moriyama S, Haneda H. Thymectomy using the subxiphoid approach. J Thorac Cardiovasc Surg 2016;152:278-9. [Crossref] [PubMed]
- Van Kolen K, Pierrache L, Heyman S, et al. Prognostic factors and genetic markers in thymoma. Thorac Cancer 2010;1:133-40. [Crossref] [PubMed]
- Detterbeck FC, Huang J. Overview. J Thorac Oncol 2011;6:S1689-90. [Crossref] [PubMed]
- Venuta F, Rendina EA, Coloni GF. Multimodality treatment of thymic tumors. Thorac Surg Clin 2009;19:71-81. [Crossref] [PubMed]
- Venuta F, Anile M, Rendina EA, et al. The value of transcapsular invasion in patients with thymoma. Arch Pathol Lab Med 2009;133:1364-5. [PubMed]
- Yano M, Sazaki H, Yukiue H, et al. Thymoma with dissemination: efficacy of macroscopic total resection of disseminated nodules. World J Surg 2009;33:1425-31. [Crossref] [PubMed]
- Nakahara K, Ohno K, Hashimoto J, et al. Thymoma: results with complete resection and adjuvant postoperative irradiation in 141 consecutive patients. J Thorac Cardiovasc Surg 1988;95:1041-7. [PubMed]
- Rea F, Marulli G, Girardi R, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg 2004;26:412-8. [Crossref] [PubMed]
- Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995;60:908-13; discussion 914. [Crossref] [PubMed]
- Quintanilla-Martinez L, Wilkins EW Jr, Choi N, et al. Thymoma. Histologic subclassification is an independent prognostic factor. Cancer 1994;74:606-17. [Crossref] [PubMed]
- Singhal S, Shrager JB, Rosenthal DI, et al. Comparison of stages I-II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg 2003;76:1635-41; discussion 1641-2. [Crossref] [PubMed]
- Weksler B, Shende M, Nason KS, et al. The role of adjuvant radiation therapy for resected stage III thymoma: a population-based study. Ann Thorac Surg 2012;93:1822-8. [Crossref] [PubMed]
- Kundel Y, Yellin A, Popovtzer A, et al. Adjuvant radiotherapy for thymic epithelial tumor: treatment results and prognostic factors. Am J Clin Oncol 2007;30:389-94. [Crossref] [PubMed]
- Ricci C, Fiorani P, Mineo TC, et al. La chirurgia riparativa nelle ostruzioni della vena cava superiore. Gazz Int Med Chir 1970;75:1551-75.
- Ricci C, Benedetti Valentini F Jr, Mineo TC, et al. Reconstruction de la veine cave supèrieure: quinze ans d’experience en utilisant different types de materiel prothetique. Ann Chir Thorac Cardiovasc 1985;39:492-5.
- Dartevelle P, Macchiarini P, Chapelier A. Technique of superior vena cava resection and reconstruction. Chest Surg Clin N Am 1995;5:345-58. [PubMed]
- De Giacomo T, Mazzesi G, Venuta F, et al. Extended operation for recurrent thymic carcinoma presenting with intracaval growth and intracardiac extension. J Thorac Cardiovasc Surg 2007;134:1364-5. [Crossref] [PubMed]
- Zhu G, He S, Fu X, Jiang G, Liu T. Radiotherapy and prognostic factors for thymoma: a retrospective study of 175 patients. Int J Radiat Oncol Biol Phys 2004;60:1113-9. [Crossref] [PubMed]
- Urgesi A, Monetti U, Rossi G, et al. Role of radiation therapy in locally advanced thymoma. Radiother Oncol 1990;19:273-80. [Crossref] [PubMed]
- Curran WJ Jr, Kornstein MG, Brooks JJ, et al. Invasive thymoma: the role of mediastinal irradiation following complete or incomplete surgical resection. J Clin Oncol 1988;6:1722-7. [Crossref] [PubMed]
- Monden Y, Nakahara K, Iioka S, et al. Recurrence of thymoma: clinicopathological features, therapy, and prognosis. Ann Thorac Surg 1985;39:165-9. [Crossref] [PubMed]
- Ciernik IF, Meier U, Lutolf UM. Prognostic factors and outcome of incompletely resected invasive thymoma following radiation therapy. J Clin Oncol 1994;12:1484-90. [Crossref] [PubMed]
- Mangi AA, Wain JC, Donahue DM, et al. Adjuvant radiation of stage III thymoma: is it necessary? Ann Thorac Surg 2005;79:1834-9. [Crossref] [PubMed]
- Yang HC, Yoon YS, Kim HK, et al. En bloc extended total thymectomy and extrapleural pneumonectomy in Masaoka stage IVA thymomas. J Cardiothorac Surg 2011;6:28. [Crossref] [PubMed]
- Rena O, Mineo TC, Casadio C. Multimodal treatment for stage IVA thymoma: a proposable strategy. Lung Cancer 2012;76:89-92. [Crossref] [PubMed]
- Lindstrom JM, Seybold ME, Lennon VA, et al. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 1976;26:1054-9. [Crossref] [PubMed]
- Romi F. Thymoma in myasthenia gravis: from diagnosis to treatment. Autoimmune Dis 2011;2011:474512 [Crossref] [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection. A series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [Crossref] [PubMed]
- Wilkins KB, Sheikh E, Green R, et al. Clinical and pathologic predictor of survival in patients with thymoma. Ann Surg 1999;230:562-72. [Crossref] [PubMed]
- Rea F, Sartori F, Loy M, et al. Chemotherapy and operation for invasive thymoma. J Thorac Cardiovasc Surg 1993;106:543-9. [PubMed]
- Fershtand JB, Show RR. Malignant tumor of the thymus gland, myasthenia gravis developing after removal. Ann Intern Med 1951;34:1025-35. [Crossref] [PubMed]
- Yamada Y, Yoshida S, Iwata T, et al. Risk factors for developing posthymectomy myasthenia gravis in thymoma patients. Ann Thorac Surg 2015;99:1013-9. [Crossref] [PubMed]
- Mori T, Nomori H, Ikeda K, et al. The distribution of parenchyma, follicles, and lymphocyte subsets in thymus of patients with myasthenia gravis, with special reference to remission after thymectomy. J Thorac Cardiovasc Surg 2007;133:364-8. [Crossref] [PubMed]
- Ponseti JM, Gamez J, Vilallonga R, et al. Influence of ectopic thymic tissue on clinical outcome following extended thymectomy in generalized seropositive nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg 2008;34:1062-7. [Crossref] [PubMed]
- Means RT Jr. Pure red cell aplasia. Blood 2016;128:2504-9. [Crossref] [PubMed]
- Bernard C, Frih H, Pasquet F, et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev 2016;15:82-92. [Crossref] [PubMed]
- Sonobe M, Nakagawa M, Ichinose M, et al. Thymoma. Analysis of prognostic factors. Jpn J Thorac Cardiovasc Surg 2001;49:35-41. [Crossref] [PubMed]
- Rosai J, Levine GD. Tumors of the thymus. In: Atlas of tumor pathology. 2nd series, fascicle 13. Washington, DC: Armed Forces Institute of Pathology, 1976.
- Pescarmona E, Rosati S, Pisacane A, et al. Microscopic thymoma: histological evidence of multifocal cortical and medullary origin. Histopathology 1992;20:263-6. [Crossref] [PubMed]
- Fukuhara M, Higuchi M, Owada Y, et al. Clinical and pathological aspects of microscopic thymoma with myasthenia gravis and review of published reports. J Thorac Dis 2017;9:1592-7. [Crossref] [PubMed]
- Furuya T, Kato D, Yamazaki S, et al. Microthymoma and microscopic thymomas associated with a thymic cyst without solid component. Gen Thorac Cardiovasc Surg 2018;66:303-6. [Crossref] [PubMed]
- Maggi G, Casadio C, Cavallo A, et al. Thymoma: results of 241 operated cases. Ann Thorac Surg 1991;51:152-6. [Crossref] [PubMed]
- Ruffini E, Mancuso M, Oliaro A, et al. Recurrence of thymomas: analysis of clinicopathologic features, treatment and outcome. J Thorac Cardiovasc Surg 1997;113:55-63. [Crossref] [PubMed]
- Nakagawa K, Asamura H, Matsuno Y, et al. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg 2003;126:1134-40. [Crossref] [PubMed]
- Okumura M, Shyomo H, Inoue M, et al. Outcomes of surgical treatment for recurrent thymic epithelial tumors with reference to World Health Organization histologic classification system. J Surg Oncol 2007;95:40-4. [Crossref] [PubMed]
- Hamaji M, Allen MS, Cassivi SD, et al. The role of surgical management in recurrent thymic tumors. Ann Thorac Surg 2012;94:247-54. [Crossref] [PubMed]
- Giaccone G, Wilmink H, Paul M, et al. Systemic treatment of malignant thymoma: a decade of experience at a single institution. Am J Clin Oncol 2006;29:336-44. [Crossref] [PubMed]
- Haniuda M, Kondo R, Numanami H, et al. Recurrence of thymoma: clinicopathological features, re-operation, and outcome. J Surg Oncol 2001;78:183-8. [Crossref] [PubMed]
- Ciccone AM, Rendina EA. Treatment of recurrent thymic tumors. Semin Thorac Cardiovasc Surg 2005;17:27-31. [Crossref] [PubMed]
- Kirschner PA. Reoperation for thymoma: report of 23 cases. Ann Thorac Surg 1990;49:550-4. [Crossref] [PubMed]
- Friedant AJ, Handorf EA, Su S, et al. Minimally invasive versus open thymectomy for thymic malignancies: systematic review and meta-analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Baldi A, et al. Recurrent intrathoracic thymomas: potential prognostic importance of cell-cycle protein expression. J Thorac Cardiovasc Surg 2009;138:40-5. [Crossref] [PubMed]
- Mineo TC, Mineo D, Onorati I, et al. New predictors of response to neoadjuvant chemotherapy and survival for invasive thymoma: a retrospective analysis. Ann Surg Oncol 2010;17:3022-9. [Crossref] [PubMed]
- Guthrie GJK, Charles KA, Roxburgh CSD, et al. The systemic inflammation-based neutrophil–lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. [Crossref] [PubMed]
- Janik S, Raunegger T, Hacker P, et al. Prognostic and diagnostic impact of fibrinogen, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio on thymic epithelial tumors outcome. Oncotarget 2018;9:21861-75. [Crossref] [PubMed]
- Yanagiya M, Nitadori JI, Nagayama K, et al. Prognostic significance of the preoperative neutrophil-to-lymphocyte ratio for complete resection of thymoma. Surg Today 2018;48:422-30. [Crossref] [PubMed]
Cite this article as: Mineo TC, Sellitri F, Ambrogi V. Thymectomy for thymoma spanning three decades at Tor Vergata thoracic surgery center. J Vis Surg 2018;4:189.


