The application of robotic surgery on the anterior mediastinal tumors
Introduction
Thymoma is a rare neoplasm arising from thymic gland, with an annual incidence of 0.15 per 100,000 person-years (1). This tumor is more frequent in adults, with a peak in the sixth decade of life (2). Frequently patients with thymoma develop a paraneoplastic syndrome, constituted in 50% of cases by myasthenia gravis (MG) (3). Thymoma is usually characterized by indolent growth, but due to its malignant potential it can invade locally and metastasize regionally (4). Surgery represents the cornerstone of treatment and the prognosis depends on the stage of the disease and on the possibility to obtain a complete surgical resection. Albeit various authors have reported similar oncologic results after the exeresis of the tumor without complete thymectomy (thymomectomy), the international thymic malignancy group (ITMIG) suggests removing thymoma, thymus and mediastinal fat (5). In fact, an extended thymectomy ensures clear margins, thus a radical resection, and prevents recurrence due to hidden multifocal cancer localized in other parts of thymic tissue. Moreover, extended thymectomy is associated with improvement of neurological symptoms in myasthenic patients and it is useful to avert the appearance of post-operative myasthenic syndrome, which arises in 10% of no-MG thymomas.
For a long period, the open approach (sternotomy or thoracotomy) has been considered the optimal surgical choice for thymectomy, in spite of this nowadays the application of minimally invasive surgery (MIS), video-assisted thoracic surgery (VATS) and robotic surgery, is increasing and several studies have demonstrated positive short-term results. Robotic surgery is associated with shorter length of stay, reduction of post-operative complications, better cosmetic result and quality of life when compared to open surgery. Thanks to its features (3D vision, magnification of images, large range of instruments movements, tremor filtration), robotic technology allows to perform the operation following the surgical principals as suggested by the NCCN guidelines. To date only a few papers have analyzed oncologic outcomes, confirming comparable results between robotic thymectomy and open approach (6,7).
Several different approaches have been described for robotic thymectomy: left side, right side, bilateral and recently single-port sub-xiphoid approach. The right-side approach is favored by a large percentage of surgeons, due to the larger space for robotic instrument provided an optimal visualization of the superior vena cava and innominate vein. Whereas other surgeons prefer the left-side approach because thymic area is better exposed and left pericardio-phrenic fat tissues can be more easily reached. In addition, given the anatomical position of the innominate vein and the deep position of the left phrenic nerve, the left approach guarantees a more radical thymectomy. In presence of a large thymoma or a tumor with an uncertain cleavage plane from surrounding structures, the procedure side is determined by the tumor location. Some authors choose a bilateral approach to treat myasthenic patients or to achieve an adequate mediastinal structure control in advanced stage tumors. Recently Takashi Suda has introduced the sub-xiphoid approach, reporting an optimal visual field between the phrenic nerves, also in upper mediastinal area (8-11).
Patient selection and workup
At the beginning of the robotic experience on anterior mediastinal lesion removal, the tumour size (<3 cm) was an important criterion for patient selection. Currently, even large thymomas are eligible for robotic exeresis and dimension is not considered an absolute contraindication to MIS. According to ITMIG recommendations, the most important key point during minimally invasive thymectomy must be the insurance of a complete resection of the thymoma, limiting the manipulation of the neoplasm and avoiding accidental injury of the capsule with consequent tumour seeding. During the removal of thymoma surgeons must guarantee a radical resection, limiting the risk of cell dissemination, following “no-touch” technique and removing the specimen with an endobag (12).
In summary, all patients with anterior mediastinal lesion without radiologic evidence of great vessels or pericardium infiltration are eligible for robotic thymectomy, regardless of the size of the tumour.
Pre-operatively all patients are evaluated by a multidisciplinary team (surgeons, neurologists and anesthesiologists) to assess the indication to surgical treatment. Therefore, the optimal surgical approach and timing are decided according to the radiological aspects of the thymoma and the clinical condition of the patient.
Blood concentration of anti-acetylcholine receptor autoantibodies (AchRAb) and muscle-specific tyrosine kinases antibodies (MuSKAb) are dosed, an accurate anamnesis and a complete neurological examination are performed to diagnose a possible MG syndrome. Immediately before the surgery, myasthenic patients are revaluated by the neurologist to ensure the optimal therapeutic window.
Pre-operative preparation
Approximately 30 minutes before surgery patients receive premedication with atropine 0.5 mg and diazepam 10 mg; in myasthenic patients diazepam is contraindicated, dexamethasone 8 mg is also administered intramuscularly to reduce worsening of neurological symptoms caused by surgical stress.
Intubation is performed with a double lumen tube, to obtain lung deflation on the side of surgical accesses.
According to our experience, we prefer left-side approach, except in case of right side thymoma. The patient is placed in a supine position with a pillow under the spine and the left arm is in a flexed and in a lower position, to fully expose the left side of the chest and to maximize the working space.
Equipment preference card
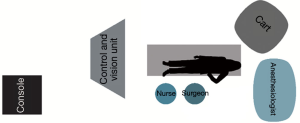
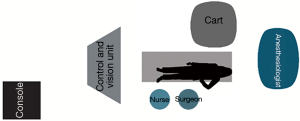
With the SI system, the surgical cart is positioned, behind the patient’s head, on the right side of the operating table, in an oblique direction (Figure 1). While using the XI system, the surgical cart is first placed from the right side of the patient, and then the optimal arms placement is obtained thanks to auto-targeting with camera pointed in jugulum area (Figure 2). With both systems the surgeon and scrub nurse work from the left side of patient, while the anesthesiologist is positioned near the head of the patient, to easily check the patient conditions or intubation when necessary.
Procedure technique
Before the exclusion of the left lung, when CO2 insufflation starts (P=5–7 mmHg), a 500 mL of preload and 5 of positive end-expiratory pressure (PEEP) are applied by the anesthesiologist in order to counteract the mediastinal shifting to right.
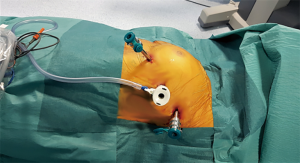
The first incision is performed in the 5th intercostal space at the anterior-axillary line and the camera is inserted to explore the chest cavity. CO2 is inflated to increase the operating space and safely perform the other two port incisions, one at the 3rd intercostal space in anterior-axillary line and the other at the 5th intercostal space in mid-clavicular line (Figure 3).
The instruments used are the Spatula (EndoWrist; Intuitive Surgical), Maryland (EndoWrist; Intuitive Surgical) and Fenestrated Bipolar forceps (EndoWrist; Intuitive Surgical),
Currently, we use a monopolar instrument (spatula) on the right hand, and a bipolar instrument (Fenestrated Bipolar forceps on the left hand. In all cases a 30° scope is used, permitting an excellent visualization of mediastinal structures.
In the left-side approach, the dissection starts inferiorly, firstly from the right side in the retrosternal area, finding the right mediastinal pleura and the right phrenic nerve, achieving a safe dissection of the right inferior horn under direct vision of the nerve. The surgeon goes then ahead on the left side, at the pericardio-phrenic angle and following the left phrenic nerve, moving upwards towards the neck until the identification of thymic superior horns. Usually, the thymic veins are isolated and separately clipped. The specimen is removed with an endoscopic bag from the cavity through a port incision. A drainage tube is inserted in left thoracic cavity
After the operation vital parameter of the patient is monitored for approximately 12 hours and generally the day after the operation the chest drain is removed, except in case of presence of air leak or of major risk of bleeding during concomitant oral anticoagulant therapy due to neoplasm lung infiltration.
During robotic thymectomy, some approach-related complications could occur.
- Intra-operative complications:
- Alterations in cardiac rhythm, due to heart stimulation during trocar insertion in the left approach or dissection of thymic tissue from pericardium.
- Hemodynamic variations that can lead to cardiac arrest caused from high CO2 pressure and/or flow.
- Bleeding from thymic vein or innominate vein. In small vessels, as in thymic vein, clips with the appropriate size must be applied, it is therefore recommended to use titanium ligation clips and to avoid Hemolok (Teleflex Medical, Research Triangle Park, NC, USA), which, due to its dimension and to the characteristics of vein wall, could dislocate, this may also occur later in time. Moreover, while isolating the thymic gland in its upper portion, it is fundamental to move forward carefulness to abstain from innominate vein lesion, favoured by the CO2-related wall collapse.
- Post-operative complications:
- Chylothorax, produced by injury of small lymphatic vessels located into perithymic fat tissue.
- Phrenic nerve paralysis, caused by inadvertent damage to nerve during tumor dissection.
Tips, tricks and pitfalls
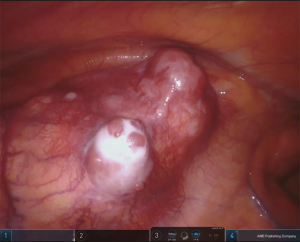
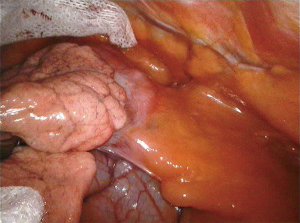
The side of the approach should be determined by the location of the thymoma in mediastinum, to guarantee a safe removal of the tumor reducing the risk of any capsular damage or of a non-radical resection, in particular in case of adhesion or infiltration (Figures 4,5).
To limit any injury of the thymoma, it is recommended to start the thymectomy from its opposite side, dissecting the tumor at the final step of procedure. It is mandatory to avoid any direct manipulation of thymoma; for this reason, it can be useful to place a gauze over the tumor to protect it from accidental lesions during the thymus dissection. In the presence of a large thymoma, which limits the vision and the control on mediastinum, the surgeon can remove firstly the whole tumor and then continue with thymectomy.
The specimen, after its introduction into the endobag, can be removed through the superior surgical access, where the intercostal space is larger allowing an easier extraction of the thymoma.
During the thymectomy, in case of difficulties in the identification of the phrenic nerve due to the presence in the anterior mediastinum of abundant fat tissue or if the thymoma is neighboring it, the DaVinci Firefly vision can be applied. This system, after intravenous injection of Indocyanine green, allows the identification of the nerve thanks to its near-infrared technology. Moreover, the use of bipolar cautery instruments is always recommended to limit accidental nerve trauma.
Conclusions
From the first robotic thymectomy performed by Yoshino in 2001 for a small thymoma, robotic surgery, thanks to its characteristics, still represents an excellent alternative to the open approach (13). In fact, taking advantage of its features, the robotic system consents to perform surgical procedures in anterior mediastinal lesions according ITMIG recommendations, in a safe manner, ensuring a minimally-invasive approach to the patients. By the increased 3D vision, it is possible to obtain during robotic unilateral approach the visualization of contralateral phrenic nerve, fundamental requisite in extended thymectomy. Furthermore, the large range of robotic instruments maneuverability assists the surgeon to reach even the deepest areas, as jugulum or costophrenic area, and to achieve an optimal control on vessels. Therefore, thanks to 3D increased vision, tremor filter and large range of available instruments, the robotic system allows to perform surgical procedures that require fine dissection of the mediastinal structures, also in case of difficulties, such as adhesion or infiltration of adjacent structures. To confirm this, in the recent years several authors have reported their experience on the application of robotic surgery in mediastinal tumor, supporting the effectiveness and safety of this approach for thymoma removal. Thus, using this innovative technology, skilled surgeons can also perform challenging operations in advanced stage thymoma, with comparable results to open surgery, guarantying to the patient a safe procedure with reduced trauma and better post-operative quality of life (Figure 6).

Acknowledgments
We thank Teresa Hung Key for linguistic accuracy checking.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Tommaso Claudio Mineo) for the series “Mediastinal Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The series “Mediastinal Surgery” was commissioned by the editorial office without any funding or sponsorship. FM is an official proctor for Intuitive Surgical. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003;105:546-51. [Crossref] [PubMed]
- de Jong WK, Blaauwgeers JL, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123-30. [Crossref] [PubMed]
- Tormoehlen LM, Pascuzzi RM. Thymoma, myasthenia gravis, and other paraneoplastic syndromes. Hematol Oncol Clin North Am 2008;22:509-26. [Crossref] [PubMed]
- Detterbeck FC, Zeeshan A. Thymoma: current diagnosis and treatment. Chin Med J 2013;126:2186-91. [PubMed]
- Nakagawa K, Yokoi K, Nakajima J, et al. Is Thymomectomy Alone Appropriate for Stage I (T1N0M0) Thymoma? Results of a Propensity-Score Analysis. Ann Thorac Surg 2016;101:520-6. [Crossref] [PubMed]
- Kneuertz PJ, Kamel MK, Stiles BM, et al. Robotic Thymectomy Is Feasible for Large Thymomas: A Propensity-Matched Comparison. Ann Thorac Surg 2017;104:1673-8. [Crossref] [PubMed]
- Marulli G, Comacchio GM, Schiavon M, et al. Comparing robotic and trans-sternal thymectomy for early-stage thymoma: a propensity score-matching study. Eur J Cardiothorac Surg 2018;54:579-84. [Crossref] [PubMed]
- Ruffini E, Filosso PL, Guerrera F, et al. Optimal surgical approach to thymic malignancies: New trends challenging old dogmas. Lung Cancer 2018;118:161-70. [Crossref] [PubMed]
- Brown LM, Louie BE. Robot-Assisted Total Thymectomy: How I Teach It. Ann Thorac Surg 2017;103:369-72. [Crossref] [PubMed]
- Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [PubMed]
- Kawaguchi K, Fukui T, Nakamura S, et al. A bilateral approach to extended thymectomy using the da Vinci Surgical System for patients with myasthenia gravis. Surg Today 2018;48:195-9. [Crossref] [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Yoshino I, Hashizume M, Shimada M, et al. Thoracoscopic thymomectomy with the da Vinci computer-enhanced surgical system. J Thorac Cardiovasc Surg 2001;122:783-5. [Crossref] [PubMed]
- Zirafa CC, Ricciardi S, Cavaliere I, et al. Robotic extended thymectomy for 5 cm thymoma adjacent to the left phrenic nerve. Asvide 2018;5:738. Available online: http://www.asvide.com/article/view/26973
Cite this article as: Zirafa CC, Ricciardi S, Cavaliere I, Davini F, Melfi F. The application of robotic surgery on the anterior mediastinal tumors. J Vis Surg 2018;4:190.