Stented versus stentless aortic valve replacement in elderly: a systematic review and meta-analysis
Introduction
Increased average life expectancy has led to an ever-greater proportion of elderly patients (1). They are more susceptible to declining functions of the body, including cognitive impairment, decreasing physical strength, relative inactivity, slow gait and falling, and many others (1). At 75 years of age, it is estimated that 4.6% of patients have severe aortic stenosis (AS) (2), which carries a poor prognosis (3). The pathophysiology accounting for the commonness of AS in the elderly involves calcification of the valve leaflets, limiting valve leaflet movements and increasing left ventricular pressure (4). Danielsen and colleagues have predicted a 2.4-fold increase in the number of patients with AS by 2040 (5). Therefore, it is important to choose the correct therapeutic option for these patients.
One of the biggest decisions for management of AS is whether to undergo aortic valve replacement (AVR), the only definitive treatment, or manage conservatively (6). Conservative management of severe AS has a mortality rate of up to 90% at 2 years (7). With increasing life expectancy, however, relative benefits and harms between surgical treatment and conservative management has become more blurred (1,2).
Surgical treatments of AS include surgical aortic valve replacement (SAVR), in which the valve may be stented or stentless (8,9). There has been much debate over their efficacy, and neither has been demonstrated to be clearly superior over the other. As such, both are currently acceptable in selected cases of severe AS (6). Stentless bioprosthesis has been reported to have better outcomes and less morbidity than the stented counterpart (6). One theory is that the sewing ring of the stented bioprosthesis impedes, by its bulk, valvular outflow, increasing left ventricular pressure and causing stress (10). In contrast, stentless valves appear to lower left ventricular mass more quickly, possibly due to less patient-prosthesis mismatch (8,11,12).
This report aims to look at the morbidity and mortality rates of stented versus stentless valve replacement in the elderly cohort.
Methods
Literature search strategy and inclusion criteria
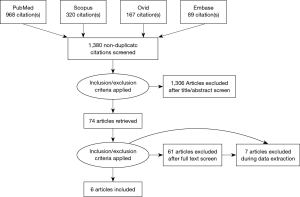
Electronic database searches were performed with PubMed, Ovid Medline, Scopus, Embase to identify all randomized and nonrandomized controlled trials comparing stentless to stented bioprosthetic valves in elderly patients undergoing AVR available up to March 2017. Limits were placed on only articles written in the English language and included elderly patients (age ≥75 years old) that underwent AVR. Search terms included aortic valve, stented, stentless, replacement, and elderly. All search terms were combined with Boolean operators and searched as both key words and MeSH terms to ensure maximal sensitivity. After excluding articles based on title or abstract, full text articles selected had reference lists searched for any potential further articles to be included in this review.
Data extraction and critical appraisal
The main outcome measures extracted included the following: in-hospital mortality, post-operative complications, stroke, and 5-year survival rate. Other data were also extracted for assessment of perioperative characteristics of patients. Quality of studies included was assessed by the Newcastle-Ottawa Scale (Table 1).
Table 1
| Author | Selection | Comparability | Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representation of patients with stented AVR | Selection of patients with stentless AVR | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Age =*; indication of surgery =* | Assessment of outcomes | Follow-up long enough for outcomes to occur | Adequacy of follow-up of cohorts | |||
| Bové |
* | ** | * | – | ** | * | * | * | ||
| Burgazli |
* | * | * | * | * | * | – | * | ||
| Doss |
* | * | * | * | ** | * | * | * | ||
| Ennker |
* | * | * | * | ** | – | * | * | ||
| Van Nooten |
* | * | * | * | * | * | * | * | ||
| Risteski |
* | * | * | * | ** | * | * | * | ||
*, equal to one point. AVR: aortic valve replacement
Statistical analysis
Standard descriptive statistics [reported as means with 95% confidence intervals (CI) where available] were used to summarize demographic and baseline data of the patients from all eligible studies. Meta-analysis of reported outcomes when reported was performed on the reported in-hospital mortality, 5-year survival rate, duration of cardiopulmonary bypass, duration of aortic cross clamp, and stroke.
Inconsistency was reported as the I2 statistic and Cochran Q statistic, and the presence of inconsistency determined the use of fixed or random effect model. Fixed effect was estimated by the Hedges-Olkin method, and random effect was estimated by the DerSimonian-Laird method. Bias was estimated by the Egger method. All statistical analysis was conducted with Review Manager Version 5.1.2 (Cochrane Collaboration, Software Update, Oxford, United Kingdom), and Stata Version 15.1 (StatCorp LLC, Texas, USA).
Results
Study demographics
A total of six comparative studies were selected through the literature (10,13-17). The search strategy is summarized by a PRISMA chart in Figure 1. As a result, a total of 1,048 patients were included in this study, of which 463 underwent stented AVR and 585 underwent stentless AVR. A summary of study characteristics is shown in Table 2.
Table 2
| Author | Year | Country | Type of study | Total number of patients | Stented (n) | Stentless (n) | Primary end points |
|---|---|---|---|---|---|---|---|
| Bové |
2006 | Belgium | Retrospective | 255 | 110 | 145 | 30-day, 1-year and 5-year mortality; patient-prosthetic mismatch; valve haemodynamic data (echocardiographic variables) |
| Burgazli |
2013 | Germany and Turkey | Prospective randomised trial | 40 | 20 | 20 | Peak and mean pressure gradients; effective orifice areas |
| Doss |
2003 | Germany | Prospective randomised trial | 40 | 20 | 20 | Postoperative, 6-month and 12-month clinical and haemodynamic outcomes |
| Ennker |
2003 | Germany | Retrospective | 519 | 242 | 277 | Operative risk; postoperative complications |
| Van Nooten |
1999 | Belgium | Prospective | 154 | 51 | 103 | Indications; mid-term clinical outcomes including mortality |
| Risteski |
2009 | Germany | Prospective randomised study | 40 | 20 | 20 | Early and mid-term postoperative improvements including regression of left ventricular hypertrophy and maximization of effective orifice area |
Perioperative results
Perioperative characteristics of included patients are summarized in Table 3. Preoperatively, patients undergoing stentless AVR generally had significantly higher left ventricular ejection fraction (LVEF; P=0.03), lower pre-morbid rates of chronic obstructive pulmonary disease (COPD; P=0.02), higher atrioventricular (AV) gradient (P<0.0001) and left ventricular end-diastolic diameter (LVEDD; P<0.0001), and lower left ventricular end-systolic diameter (LVESD; P<0.0001).
Table 3
| Variables | Stented | Stentless | P |
|---|---|---|---|
| No. of patients | 463 | 585 | – |
| Mean age (years) | 77.6±11 | 76.9±9 | 0.25 |
| Male (%) | 45.5 | 44.6 | N/A |
| LVEF (%) (mean ± SD) | 65.1±12 | 66.6±11 | 0.03 |
| IHD/CAD (%) | 51.5 | 52 | 0.35 |
| HTN (%) | 50.4 | 52.1 | 0.41 |
| DM (%) | 30.2 | 29.1 | 0.46 |
| COPD (%) | 17.4 | 12.6 | 0.02 |
| AV gradient (mean ± SD) | 37.6±11 | 45.2±14.3 | <0.0001 |
| BSA (mean ± SD) | 1.8±0.6 | 1.75±0.5 | 0.14 |
| LVESD, mm (mean ± SD) | 35±2 | 32±3 | <0.0001 |
| NYHA III/IV (%) | 81.75 | 80.08 | 0.5 |
| Aortic stenosis (%) | 96 | 96.4 | N/A |
| LVEDD, mm (mean ± SD) | 46±3 | 48±4 | <0.0001 |
| Mean follow-up, years (mean ± SD) | 4.14±0.95 | 4.90±1.75 | N/A |
| AVA, cm2 | 1.23±0.2 | 1.23±0.3 | 0.5 |
| Operative data | |||
| Size of valve used, mm (mean ± SD) | 23.45±1.85 | 23.98±1.90 | <0.0001 |
| CPB time, mins (mean ± SD) | 101.95±23.73 | 122.95±27.28 | <0.0001 |
| Aortic cross clamp time, mins (mean ± SD) | 69.53±16.22 | 85.72±19.65 | 0.024 |
| Concomitant procedures (%) | 45.4 | 40.4 | N/A |
| Post-operative data | |||
| AF (%) | 5.9 | 8.7 | <0.001 |
| Acute MI/cardiac events (%) | 0.6 | 2.05 | 0.01 |
| Stroke (%) | 4.35 | 3.88 | 0.50 |
| PPM (%) | 1 | 3.45 | <0.001 |
| Re-operation for bleeding (%) | 3.5 | 3.45 | 0.3 |
| In-hospital mortality (%) | 7.2 | 2.46 | <0.0001 |
| 1-year mortality (%) | 27.5 | 10.7 | 0.01 |
| 5-year mortality (%) | 24 | 16 | 0.835 |
| Mean AV gradient, mmHg (mean ± SD) | 7.28±3.75 | 8.40±3.56 | <0.0001 |
| Effective orifice area, cm (mean ± SD) | 1.41±0.47 | 2.07±0.52 | <0.0001 |
| LVEF, % (mean ± SD) | 66.2±10.5 | 67.6±8.7 | 0.01 |
| Echo findings at 6 months | |||
| Mean AV gradient, mmHg (mean ± SD) | 6.55±2.33 | 7.44±4.90 | 0.0003 |
| Effective orifice area, cm (mean ± SD) | 1.92±0.81 | 1.83±0.64 | 0.04 |
| LVEF, % (mean ± SD) | 64.7±11.3 | 66.6±8.2 | 0.001 |
| LV mass index (g/m2) | 120±27.2 | 114±34.1 | 0.002 |
N/A, not applicable; LVEF, left ventricular ejection fraction; IHD, ischaemic heart disease; CAD, coronary artery disease; HTN, hypertension; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; AV, atrioventricular; BSA, body surface area; LVESD, left ventricular end-systolic diameter; NYHA, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter; AVA, left ventricular end-diastolic diameter; AF, left ventricular end-diastolic diameter; PPM, patient-prosthesis mismatch.
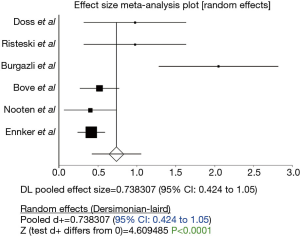
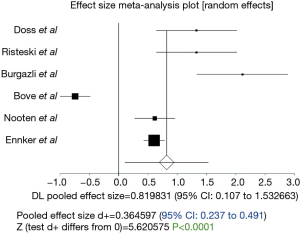
The duration of cardiopulmonary bypass was significantly longer in stentless AVR (P<0.0001; 95% CI: 0.424–1.05, Figure 2). However, Egger’s test revealed significant bias (P=0.03) and thus this result should be treated with caution. Similarly, duration of aortic cross clamping was significantly longer in stentless AVR (P<0.0001; 95% CI: 0.237 to 0.491, Figure 3).
Postoperative data showed significantly higher incidences of atrial fibrillation in patients undergone stentless procedure, as well as significantly higher rates of acute myocardial ischaemia/cardiac events (P=0.01), there was also higher rate of patient-prosthesis mismatch (PPM; P<0.0001). On echocardiography at 6 and 12 months, stentless AVR was observed to be associated with higher mean AV gradient (P<0.0001 and P=0.0003 respectively), larger effective orifice area (P<0.0001 and P=0.04 respectively) and higher LVEF (P=0.01 and P=0.001 respectively). Stentless AVR was also associated with lower left ventricular mass index at 12 months (P=0.002).
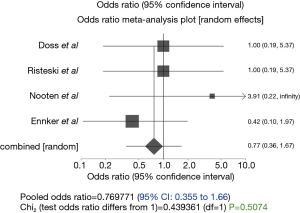
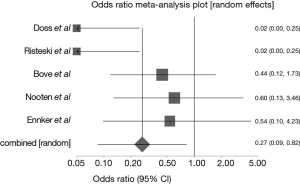
There was no observable difference in the incidence of stroke between the two groups of patients (P=0.5074; 95% CI: 0.35 to 1.66, Figure 4). In-hospital mortality was significantly lower in the stentless group (P<0.0001, 95% CI: 0.132–0.479, Figure 5), similarly, all-cause mortality at 1 year was lower in the stentless group (P=0.01).
In contrast to in-hospital mortality, there was no difference in the 5-year mortality between the two groups of patients (P=0.835, 95% CI: −0.0298 to 0.024, Figure 6).
Postoperative echocardiographic findings
While in-hospital, the mean AV gradient was lower in patients that underwent stented AVR (7.28±3.75 vs. 8.40±3.56 mmHg, P<0.0001), on the contrary, stentless group of patients had a larger effective orifice area (1.41±0.47 vs. 2.07±0.52 cm, P<0.0001). A repeated echocardiogram at six months post discharge showed that the mean AV gradient remained lower in stented group of patients (6.55±2.33 vs. 7.44±4.90 mmHg, P=0.0003), interestingly the effective orifice area has improved and showed better results in stented group (1.92±0.81 vs. 1.83±0.64 cm, P=0.04).
Discussion
Aortic valve stenosis (AS) is the most common isolated valvular disease (18). The natural history of AS, first proposed by Ross and Braunwald (19) in 1968, has been confirmed in numerous studies over the last few decades. It is characterized by a benign course if asymptomatic, but has a rapid mortality rate once symptomatic. Although Ross and Braunwald proposed the middle age as the time of such symptomatic demarcation, patients now often present later in the seventh to ninth decades, and invariably have dire outcome unless AVR is performed (20). This recognition has prompted the growing numbers of patients referred for SAVR (20). The prevalence of moderate or severe AS in patients ≥75 years old is 2.8%, while at the same time, approximately 50% of patients with severe AS are referred for surgery and 40% undergo SAVR (21).
In our review, we compared these two types of valves in terms of their performances in patients older than 75 years old. The operative times required for the implantation of the prosthesis were the first points of comparison. It was demonstrated that there was significantly longer bypass (P<0.0001; 101.95±23.73 min in stented group vs. 122.95±27.28 min in stentless group) and aortic cross clamp time (P<0.0001, 95% CI: 0.237 to 0.491; 69.53±16.22 min in stented group vs. 85.72±19.65 min in stentless group) in the stentless group, reflecting its higher technicality. Similarly, Van Nooten et al. (16) found that the required cross clamp-time for isolated AVR was 70 min for stemless versus 58 min for stented valves. Burgazli et al. (10) also reported cross clamp time (81.7±16.17618 min in stentless group vs. 53±8.8674 min in stented group) and cardiopulmonary by-pass times (106.15±24 min in stentless group vs. 68.20±8.8294 min in stented group) being significantly longer in stentless group (16).
Despite the significant difference regarding the operative times, it was revealed that in-hospital mortality was lower in stentless valves (P<0.0001; 95% CI of fixed effect: 0.132–0.479; 2.46% in stentless group vs. 7.2% in stented group). Many authors correlated this with the fact that patients undergoing AVR with a stented valve were generally sicker, while AVR deploying a stentless valve was a “more elective” surgical case. Another interesting finding was that a number of studies (14,16), regardless of whether there was equal or superior in-hospital mortality of one of the two types of valves, all 30-day mortalities were believed to be unrelated to the valve condition and post-mortem examination excluded valve dysfunction.
In contrast, our review did not identify any differences in the 5-year survival between the two groups and the survival curves were found to be within the expected survival of the health population. Del Rizzo et al. (22) compared stentless and stented xenografts with particular interest for different age group and found a survival gain in favour of stentless valves at 5 years but only in the younger patients. However, in the group with patients aged more than 70 years, the prosthetic design had fewer effects on late survival than patients’ age.
In addition, this review did not find the incidences of stroke between the two groups of patients significantly different (P=0.5074; 95% CI of fixed effect: 0.35 to 1.66; 4.35% in stented group vs. 3.88% in stentless group). This is consistent with the literature (16).
The choice between mechanical and bioprosthetic valves in AVR remains controversial. Both the 2017 AHA/ACC guideline (23) and 2017 ESC/EACTS guideline (24) emphasized patient-centred decision with consideration of patients’ personal preferences and multiple other factors, mostly related to the different requirements for anticoagulation, rates of structural deterioration, and risks of intervention. Both guidelines considered preoperative anticoagulant therapy, longevity, and high risk in re-interventions as factors in favour of mechanical prostheses. The 2017 AHA/ACC guideline also specified small aortic root size for AVR as a favourable factor for mechanical prostheses. Contraindications for anticoagulation and desire for pregnancy are strong, if not absolute indications for bioprostheses. Other favourable factors include high risks of anticoagulation, limited access to anticoagulation monitoring, and accessible surgical centres with low re-operative mortality rates. Age is consistently important in the decision-making, but the suggested cut-offs vary: the 2017 AHA/ACC guideline recommended mechanical prostheses for patients under the age of 50 and bioprostheses for those above the age of 70, while the 2017 ESC/EACTS guideline recommended mechanical prostheses or patients under the age of 60 and bioprostheses for those above the age of 65. Other factors ought to be considered for patients in between the abovementioned cut-off ages. These recommendations were made mainly on the grounds of the age-dependent incidences of structural prosthetic deterioration and risks in re-intervention as reported by literature.
As such, for elderly AS patients, the main question that arises in clinical practice is which type of bioprostheses, stentless or stented, is superior. This has not been commented in either of the abovementioned guidelines. Long-term studies comparing stentless and stented valves in elderly AS patients remain scarce, despite the long history of their clinical usage, which may be traced back to 1972 when Cohn and colleagues first deployed porcine xenografts in SAVR (25). The use of stentless valves has increased steadily in the last decade due to their commercial availability, improved durability and lower prosthetic mismatches (10,15,26). As such, the choice between stentless and stented valves is a pertinent issue that warrants further, more comprehensive investigations. The scope of investigation may be expanded beyond simply the choice of valves for elderly patients, but also the possibility of using bioprostheses in younger patients. Given the improving haemodynamics and thus durability of these valves (27), it may be possible to lower the recommended cut-off age for bioprostheses, hence allowing more and younger patients to enjoy the rest of their life free of anticoagulants and a low risk of re-intervention at least similar to that of mechanical prostheses.
This review carries the limitation that haemodynamic comparison of the two types of valves was not included. Borger et al. (28), during a midterm follow-up in a large number of patients underwent AVR, reported that the stentless bioprostheses were haemodynamically superior to stented valves. However, strong evidences of better haemodynamic and clinical performance have not been repeated in the literature. Risteski et al. (17) noticed incomplete regression of left ventricular mass in both groups, demonstrating the inevitable influence of other factors like age, hypertension and gender. Another interesting point, as highlighted by Jin et al. (29), was that the left ventricular mass regression was completed at 6 months postoperatively in patients with stentless valves, whereas stented valves did not achieve completion of such even after 12 months. Moreover, a lot of interest has been addressed to the concept of PPM as a factor that could jeopardize the regression of left ventricular hypertrophy despite having undergone AVR (30). However, the clinical impact of this issue remains controversial.
Limitations
The results from this meta-analysis need to be considered carefully as there are several limiting factors. Firstly, majority of the data analysed comes from retrospective cohort studies while the randomized studies have low number of patients that doesn’t power the studies enough to give a strong statement in comparing the outcomes, therefore this can form a confounding factor that can limit the interpretation of the results. Secondly, in the retrospective studies there is no mention of the experience of the operating surgeon or the decision process of choosing the type of valve, these two factors can contribute significantly to the results and again it limits the outcomes in this study. Furthermore, there is also lack of intra-operative echocardiography imaging which can also help in assessing the aortic orifice and potentially assist in deciding choice of the valve. Fourthly, the data are extremely heterogeneous and there are multiple confounding factors, including patient selection, especially in the retrospective cohort studies. Additionally, publication bias may have influenced the results from our study, as observational studies with a poor outcome may not have been published their results in full details. Finally, there is lack of long term data, the mean follow-up is limited to six months for echocardiographic findings and this represent a very short-term results which can’t be used reliably as a base for proper follow up and interpretation of the results.
Conclusions
The outcome from this study showed that although patients with stentless AVR had longer operative data, they showed a better operative and 1-year mortality rates, while the rate of 5-year mortality were higher in stented group but this didn’t reach statistical significance. However, the echocardiographic findings are mixed and the results are controversial, never-mind the results are limited to only 5 years, therefore long-term data required to give a better understanding of superiority of each valve over the other.
Acknowledgments
This abstract has been presented as oral presentation in the 26th Annual Meeting of the Asian Society of Cardiovascular and Thoracic Surgery, Moscow, Russia.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.08.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mack M. Frailty and aortic valve disease. J Thorac Cardiovasc Surg 2013;145:S7-10. [Crossref] [PubMed]
- Martínez-Sellés M, Gómez Doblas JJ, Carro Hevia A, et al. Prospective registry of symptomatic severe aortic stenosis in octogenarians: A need for intervention. J Intern Med 2014;275:608-20. [Crossref] [PubMed]
- Alsara O, Alsarah A, Laird-Fick H. Advanced age and the clinical outcomes of transcatheter aortic valve implantation. J Geriatr Cardiol 2014;11:163-70. [PubMed]
- Aronow WS. Valvular aortic stenosis in the elderly. Cardiol Rev 2007;15:217-25. [Crossref] [PubMed]
- Danielsen R, Aspelund T, Harris TB, et al. The prevalence of aortic stenosis in the elderly in Iceland and predictions for the coming decades: the AGES-Reykjavík study. Int J Cardiol 2014;176:916-22. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [Crossref] [PubMed]
- Dunning J, Graham RJ, Thambyrajah J, et al. Stentless vs. stented aortic valve bioprostheses: a prospective randomized controlled trial. Eur Heart J 2007;28:2369-74. [Crossref] [PubMed]
- Ghoneim A, Bouhout I, Demers P, et al. Management of small aortic annulus in the era of sutureless valves: A comparative study among different biological options. J Thorac Cardiovasc Surg 2016;152:1019-28. [Crossref] [PubMed]
- Kunihara T, Schmidt K, Glombitza P, et al. Root replacement using stentless valves in the small aortic root: a propensity score analysis. Ann Thorac Surg 2006;82:1379-84. [Crossref] [PubMed]
- Burgazli KM, Mericliler M, Erenturk S, et al. Early postoperative hemodynamic performances of stented versus stentless aortic xenografts in aortic valve replacement in elderly patients: a comparative study. Eur Rev Med Pharmacol Sci 2013;17:1894-900. [PubMed]
- Shalabi A, Spiegelstein D, Sternik L, et al. Sutureless Versus Stented Valve in Aortic Valve Replacement in Patients with Small Annulus. Ann Thorac Surg 2016;102:118-22. [Crossref] [PubMed]
- Maselli D, Pizio R, Bruno LP, et al. Left ventricular mass reduction after aortic valve replacement: homografts, stentless and stented valves. Ann Thorac Surg 1999;67:966-71. [Crossref] [PubMed]
- Bové T, Van Belleghem Y, François K, et al. Stentless and stented aortic valve replacement in elderly patients: Factors affecting midterm clinical and hemodynamical outcome. Eur J Cardiothorac Surg 2006;30:706-13. [Crossref] [PubMed]
- Doss M, Martens S, Wood JP, et al. Performance of stentless versus stented aortic valve bioprostheses in the elderly patient: a prospective randomized trial. Eur J Cardiothorac Surg 2003;23:299-304. [Crossref] [PubMed]
- Ennker J, Rosendahl U, Ennker IC, et al. Risk in elderly patients after stentless versus stented aortic valve surgery. Asian Cardiovasc Thorac Ann 2003;11:37-41. [Crossref] [PubMed]
- Van Nooten G, Caes F, François K, et al. Stentless or stented aortic valve implants in elderly patients? Eur J Cardiothorac Surg 1999;15:31-6. [Crossref] [PubMed]
- Risteski PS, Martens S, Rouhollahpour A, et al. Prospective randomized evaluation of stentless vs. stented aortic biologic prosthetic valves in the elderly at five years. Interact Cardiovasc Thorac Surg 2009;8:449-53. [Crossref] [PubMed]
- Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol 2014;30:962-70. [Crossref] [PubMed]
- Ross J Jr, Braunwald E. Aortic stenosis. Circulation 1968;38:61-7. [Crossref] [PubMed]
- Bonow RO, Greenland P. Population-wide trends in aortic stenosis incidence and outcomes. Circulation 2015;131:969-71. [Crossref] [PubMed]
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation 2013;127:e6-e245. [Crossref] [PubMed]
- Del Rizzo DF, Abdoh A, Cartier P, et al. The effect of prosthetic valve type on survival after aortic valve surgery. Semin Thorac Cardiovasc Surg 1999;11:1-8. [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Cohn LH, Sanders JH Jr, Collins JJ Jr. Aortic valve replacement with the Hancock porcine xenograft. Ann Thorac Surg 1976;22:221-7. [Crossref] [PubMed]
- Kobayashi J. Stentless aortic valve replacement: an update. Vasc Health Risk Manag 2011;7:345-51. [Crossref] [PubMed]
- Milano AD, Blanzola C, Mecozzi G, et al. Hemodynamic performance of stented and stentless aortic bioprostheses. Ann Thorac Surg 2001;72:33-8. [Crossref] [PubMed]
- Borger MA, Carson SM, Ivanov J, et al. Stentless aortic valves are hemodynamically superior to stented valves during mid-term follow-up: a large retrospective study. Ann Thorac Surg 2005;80:2180-5. [Crossref] [PubMed]
- Jin XY, Zhang ZM, Gibson DG, et al. Effects of valve substitute on changes in left ventricular function and hypertrophy after aortic valve replacement. Ann Thorac Surg 1996;62:683-90. [Crossref] [PubMed]
- Blais C, Dumesnil JG, Baillot R, et al. Impact of valve prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation 2003;108:983-8. [Crossref] [PubMed]
Cite this article as: Harky A, Chan JSK, Ahmad M, Froghi S, Rimmer L, Bashir M. Stented versus stentless aortic valve replacement in elderly: a systematic review and meta-analysis. J Vis Surg 2018;4:201.