Supine awake subxiphoid lung resection in a severe obese patient with idiopathic pulmonary fibrosis
Case presentation
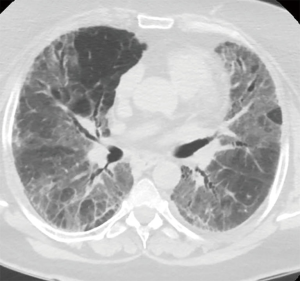
A 57-year-old woman, former smoker (30 pack-years), was referred to a respiratory department because of rapidly worsening of the respiratory symptoms in a patient with a previous diagnosis of idiopathic pulmonary fibrosis (IPF). At the beginning of 2017, during the preoperative study done for bariatric surgery (BMI 45 kg/m2). The high-resolution computed tomography (HRCT) scan shows bilateral, peripheral and subpleural areas of ground glass opacities without clear signs of honeycombing. She denied any diseases, except for severe obesity, insulin dependent diabetes and essential hypertension. Respiratory function tests were normal, with a moderate reduction of the diffusion lung CO (DLCO). The patient was tested for autoimmunity, resulting lightly positive for anti-nuclear (ANA) and anti-smooth muscle (SMA) antibodies. The patients were discharged without a definitive diagnosis with oral corticosteroids. She was stable until February 2018, when she was admitted to our Pulmonology Intensive Unit because of acute exacerbation (AE) of IPF requiring non-invasive ventilation (NIV) to achieve acceptable respiratory gas exchange.
After clinical stabilization with a bolus of steroids and high-flow nasal cannula oxygen delivery (16 L/min FiO2 60% to achieve 88–90% of peripheral saturation at rest) a new HRTC scan showed increased areas of ground glass opacity admixed with reticular abnormality diffused to lower and middle bilateral lung fields without honeycombing (Figure 1), a transthoracic echocardiographic study was performed, excluding the presence of pulmonary hypertension. After multidisciplinary meeting the temporary diagnosis was IPF inconsistent with usual interstitial pneumonia (UIP) and thus an urgent surgical lung biopsy was requested. Pulmonary function tests were not performed due the critical conditions of the patient.
Technique (Figures 2-5)


No premedication was used because of severe respiratory impairment.
In the operating room, the patient was monitored with 5 leads electrocardiography, pulse oximetry, invasive arterial blood pressure trough the right radial artery and two 16G vascular accesses for liquid and drugs infusion.
The anesthesiologic strategy agreed with the surgical team provided epidural analgesia and mild sedation with propofol in order to preserve spontaneous breathing and maintain cooperative interaction between the patient and the medical staff.
With the patient in sitting position, the thoracic epidural was placed in the T6–T7 space, with the ultrasound-assisted technique and the catheter was placed 5 cm from the entry point. The catheter suction test was negative for liquor aspiration and administration of 2 mL lidocaine 2% did not cause paresthesia (Figure 4).
A bolus of 3 mL chirocaine 0.5% and sufentanil 10 µg was administered through the epidural catheter twenty minutes before the surgical incision.
Epidural top ups bolus of 2 mL of 0.5% chirocaine and sufentanil 5 µg were given when heart rate and systemic blood pressure increased 20% from baseline.
In order to prevent the cough reflex, the oral cavity was nebulized with lidocaine 2%, 15 L/min oxygen was administered to the patient by Venturi mask in order to maintain 88–90% peripheral oxygen saturation.
According with the surgical team, surgery was performed with the patient in supine position in order to proceed with emergency intubation if needed.
Obviously, in the operating room all devices for emergency intubation were present as well as difficult airways management devices.
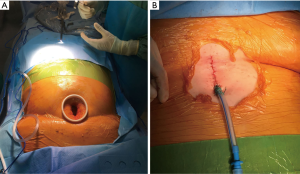
A 4-cm vertical skin incision was made above the xiphoid process (Figure 2A). The linea alba was cut in the middle for 3 to 4 cm and the rectus abdomen muscles were detached from the xiphoid process. A blunt dissection was performed using a finger to create a space under the xiphoid process toward the right chest cavity and opening the right mediastinal pleura. An incision protector (Alexis® XS, Applied Medical) was placed and a 10-mm 30° thoracoscope was inserted through the single port. Once vision of the thoracic cavity was obtained, the hole in the right mediastinal pleura was enlarged using a harmonic scalpel (Ethicon, Inc., Somerville, NJ, USA). The stapler (Endo-GIATM Ultra-Universal Standard, Covidien Products, purple reloads with tri-stapleTM Technology) was inserted from the single incision below the camera and three wedge resections of at least 3-cm of diameter were performed from the upper, the middle and the lower lobes in areas of diseased lung according to the HRTC (Figure 5). The lung collapse was excellent during the whole procedure.
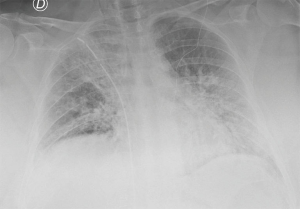
The patient well tolerated the surgical procedure and vital signs were stable during surgery. Oxygenation remained satisfactory throughout the procedure, the lowest value was 83% on peripheral saturation corrected with modifying Venturi mask oxygen delivery to keep arterial oxygen saturation at least 88%. Permissive hypercapnia was accepted monitoring the blood pH. Upon completion of surgery a 24-fr chest drain was placed under direct vision toward the apex of the right chest connected to a digital draining system (ThopazTM, Medela Italia Srl) (Figure 2B). At the end of the operation we asked the patient to take several deep breaths to improve lung re-expansion. Post-operative chest X-ray was “normal” (Figure 3). The operative time was 45 minutes.
At the end of the surgery, the patient was transferred to the ICU for monitoring. The continuous epidural infusion with 0.125% chirocaine and sufentanil 0.25 µg/mL through the elastomeric pump (6 mL/h) began two hours after surgical incision and continued for the following 72 hours. Paracetamol 1 g 3 times/day was added in the postoperative period. Total blood loss was 120 mL without air leak. The chest tube was removed in post-operative day (POD) 1 and the patient was transferred to the pneumology department in POD 2. The post-operative period was uneventful, no complications related with the procedure were detected and no signs of AE of IPF were documented. The final pathological examination excluded UIP and revealed an interstitial lung disease with NSIP (non-specific interstitial pneumonia) fibrosing pattern, a diffuse interstitial pneumonia-like reaction and several bronchiectasis.
The patient was then treated with high doses of corticosteroids and azathioprine (100 mg) with improvement of the respiratory failure and she was discharged on oxygen therapy (5 L) on the 27th POD.
Discussion
IPF represents a heterogeneous group of disorders characterized by the progressive deposition of fibrotic tissue in the lungs and overall poor prognosis. The diagnosis of different subtypes of IPF and thus the institution of the correct therapy requires a multidisciplinary team to interpret jointly different clinical scenarios radiological pathway, and histological samples (3).
The 2011 guidelines on diagnosis for IPF (4) define three diagnostic categories on HRCT appearance: UIP, possible UIP and inconsistent with UIP. The same guidelines consider surgical lung biopsy necessary when the HRCT pattern is indeterminate or inconsistent with UIP or when the clinical history of the patient is suggestive for an alternative diagnosis. Indeed, patients with different disorders that mimic IPF, in particular UIP subtype, could have a better prognosis or require different therapies.
Unfortunately, surgical lung biopsy in patients with IPF is burdened by a high morbidity and mortality (5). A larger review conducted from 1997 to 2008 in the UK reported a 30-day mortality of 2.4% and a 90-day mortality of 3.9% after surgical lung biopsy in IPF patients (6). Nowadays, video-assisted thoracoscopic surgery (VATS) under general anesthesia is the gold standard procedure for surgical lung biopsy (4,7). Nonetheless, post-operative mortality remains significant ranging between 4% to 15%, despite minimally invasive approaches (3,5,6,8). AE of IPF, characterized by progressive respiratory failure, was the most frequent cause of death after surgery (9). The pathogenesis of AE is still unknown, but surgical manipulation of the lung, mechanical ventilation with lung overdistension, high inspired oxygen fraction inhalation and postoperative infections are considered significant risk factors for AE precipitation (9,10). Moreover, some patient’s characteristics are related with even higher risk of AE such as low preoperative DLCO, UIP pattern, extreme body mass index, urgency status, etc. (9,10).
Our patient was particularly challenging first of all because of the rapid functional deterioration with DLCO less than 50%, high flow oxygen administration (16 L/min FiO2 60%), prolonged hospitalization with total nurse assistance, insulin dependent diabetes and severe obesity (BMI >43 kg/m2).
Hutchinton et al. reported a 16% postoperative mortality in patients who underwent non-elective lung biopsy (8), moreover, severe obesity is a well-known risk factor for complication after lung surgery (11). To limit the operative risk and in particular the risk of AE, we decided to perform the operation in an awake fashion without the patient’s intubation. This technique has been recently introduced in the clinical practice for different thoracic surgery procedures with excellent results (12). Avoiding intubation has potential benefits including faster recovery, fewer postoperative complications and shorter hospital stays. Especially in IPF patients avoid intubation also free from pressure induced lung injuries during single lung ventilation and exposure to high oxygen concentration (7,12,13). Moreover, obese patients with IPF have a very severe restrictive pulmonary function, thus avoiding muscle relaxation and high dose of opioids further help for a faster postoperative recovery. The use of regional anesthesia in awake non-intubated patients optimize pain control and might have contributed to minimize operative risk. Intraoperative mild sedation is important to treat anxiety and help to tolerate impaired oxygenation related to open surgical pneumothorax that induces paradoxical ventilation and intermittent partial exhaled gases rebreathing. Unconsciousness and amnesia also are favorable effects of sedation (7,13). Generally, this surgical technique is performed in lateral decubitus as in standard VATS surgery (12). In our case we decided to perform VATS resection on supine decubitus through a subxiphoid approach. The subxiphoid access is a recently introduced technique for lung and thymic surgery (14). The main advantage of uniportal approach is to further reduce post-operative pain and the risk of intercostal nerve damage secondary to intercostal port placement (14,15). Our choice was precisely to reduce post-operative pain and drugs use, in particular opioids, as much as possible. Indeed, post-operative pain in patients with extremely high BMI and with IPF and thus with a severe restrictive lung physiology could further contribute to impaired oxygenation. The choice of supine decubitus was based on two aspects. First of all, we were conscious of the high-risk of procedural failure with necessity of emergent orotracheal intubation. In this case the intubation on lateral decubitus could be very complex for the anesthetist due to the patient's soma and his respiratory tract. Indeed, obesity is considered in previous reports (7,13) a contraindication for awake VATS, mainly for the risk of emergent intubation and difficult airway management. The supine position is obviously much more favorable for tracheal intubation or supraglottic airway management. From the surgical point of view, there are some difficulties to reach the chest cavities in obese patients through subxiphoid access, but in our opinion, this alone is not an absolute contraindication. Second, the lateral decubitus on the “non-affected” site in a severe obese patient further impairs chest ventilation and gas exchange with high re-breathing ratio with worse hypercapnia, and last but not least, the supine position is well tolerated from an awake patient. Obviously, the supine position is not indicated if a major or anatomical lung resection is required.
In conclusion, a multidisciplinary approach is mandatory for the management of IPF patients, in particular when surgical lung biopsy is necessary. The surgeon and anesthetist must try to minimize surgical and anesthetic stress in order to reduce the risk of postoperative morbidity and mortality in particular AE. The awake non-intubated uniportal subxiphoid approach could be very promising technique to reduce mortality after lung biopsy for IPF. In our high-risk patient we find the supine position very useful to improve the safety of the procedure.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.09.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dell'Amore A, Campisi A, Dal Checco E, et al. The thoracic epidural catheter was placed before starting operation. Asvide 2018;5:789. Available online: http://www.asvide.com/article/view/27483
- Dell'Amore A, Campisi A, Dal Checco E, et al. Surgical procedure. Asvide 2018;5:790. Available online: http://www.asvide.com/article/view/27484
- Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med 2018;6:138-53. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Plönes T, Osei-Agyemang T, Elze M, et al. Morbidity and mortality in patients with usual interstitial pneumonia (UIP) pattern undergoing surgery for lung biopsy. Respir Med 2013;107:629-32. [Crossref] [PubMed]
- Hutchinson JP, McKeever TM, Fogarty AW, et al. Surgical lung biopsy for the diagnosis of interstitial lung disease in England: 1997-2008. Eur Respir J 2016;48:1453-61. [Crossref] [PubMed]
- Pompeo E, Rogliani P, Cristino B, et al. Awake thoracoscopic biopsy of interstitial lung disease. Ann Thorac Surg 2013;95:445-52. [Crossref] [PubMed]
- Hutchinson JP, Fogarty AW, McKeever TM, et al. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016;193:1161-7. [Crossref] [PubMed]
- Ryerson CJ, Cottin V, Brown KK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: shifting the paradigm. Eur Respir J 2015;46:512-20. [Crossref] [PubMed]
- Yano M, Sasaki H, Moriyama S, et al. Post-operative acute exacerbation of pulmonary fibrosis in lung cancer patients undergoing lung resection. Interact Cardiovasc Thorac Surg 2012;14:146-50. [Crossref] [PubMed]
- Williams T, Gulack BC, Kim S, Fernandez FG, Ferguson MK. Operative Risk for Major Lung Resection Increases at Extremes of Body Mass Index. Ann Thorac Surg 2017;103:296-302. [Crossref] [PubMed]
- Hung WT, Hsu HH, Hung MH, et al. Nonintubated uniportal thoracoscopic surgery for resection of lung lesions. J Thorac Dis 2016;8:S242-50. [PubMed]
- Zheng H, Hu XF, Jiang GN, et al. Nonintubated-Awake Anesthesia for Uniportal Video-Assisted Thoracic Surgery Procedures. Thorac Surg Clin 2017;27:399-406. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Jutley RS, Khalil MW, Rocco G. Uniportal vs standard three-port VATS technique for spontaneous pneumothorax: comparison of post-operative pain and residual paraesthesia. Eur J Cardiothorac Surg 2005;28:43-6. [Crossref] [PubMed]
Cite this article as: Dell’Amore A, Campisi A, Dal Checco E, Guerrieri A, Barbera N. Supine awake subxiphoid lung resection in a severe obese patient with idiopathic pulmonary fibrosis. J Vis Surg 2018;4:205.