
Antegrade type A aortic dissection under endoscopic vision during minimally invasive mitral valve repair: a case report
Introduction
Minimally invasive mitral valve surgery (MIMVS) has proven to be a safe alternative approach to sternotomy for surgical correction of mitral valve disease (1). Since valve exposure is achieved through a right anterolateral mini-thoracotomy and cardiopulmonary bypass (CPB) is established peripherally (usually in the femoral vessels), this procedure carries different potential complications than a conventional approach. As retrograde perfusion is used, an assessment of the abdominal aorta and peripheral vessels should be performed in order to prevent retrograde dissection, which is one of the most feared complications in MIMVS. However, we present a case of antegrade dissection during MIMVS in a patient with a normal ascending aortic diameter.
Case presentation (Figures 1-3)
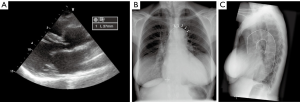


A 68-year-old female patient was referred to our centre for surgical correction of severe mitral valve regurgitation (MR). Transthoracic echocardiography (TTE) revealed a dilated left ventricle [left ventricular end-diastolic diameter (LVEDD) 68 mm] with an ejection fraction of 35% without significant aortic dilatation (sinuses of Valsalva 41 mm, reference value 29–45 mm, Figure 1A) (4). The patient was considered eligible for a minimally invasive mitral valve repair under endoscopic vision.
First, port-access incision was performed at 4th intercostal space and the pleural space was evaluated for potential adhesions before administration of unfractionated heparin for extracorporeal circulation. CPB was established in the right groin, using a femoral venous (25 Ch) and femoral arterial cannula (19 Ch) under transoesophageal echocardiographic (TOE) guidance.
Subsequently, the pericardium was opened 3 cm above the phrenic nerve and three pericardial sutures were placed. In order to achieve aortic occlusion with a transthoracic clamp, the transverse sinus was evaluated for any adhesions using forceps and a suction tube. During this standard manoeuvre a large, antegradely moving hematoma formed around the ascending aorta (Figure 2). Transoesophageal echocardiography confirmed the presence of a dissection flap (Figure 2).
An emergency sternotomy was performed. Although the ascending aorta did not seem dilated, a remarkable elongation of the aorta was observed, which was also present on preoperative chest X-ray in retrospect (Figure 1B). A left vent was placed and the patient was cooled to 25 degrees Celsius. Cardioplegia was administered selectively in both coronary ostia (St. Thomas crystalloid, 1,100 mL) after clamping and opening the aorta. The entry tear was located in the ascending aorta anteriorly opposite to where the aorta was manipulated. As the antegrade hematoma development occurred without the presence of high line pressures during establishment of CPB, antegrade type A aortic dissection (TAD) was suspected. First, a supracoronary ascending aortic replacement was performed using a 30-mm Gelweave prosthesis. During cooling to 25 degrees, the mitral valve was repaired by placing 2 pairs of neochords at the P2 segment and by implantation of a 34-mm annuloplasty ring (Carpentier Edwards Physio 4450)
Finally, a partial arch replacement was performed in circulatory arrest (18 minutes) using an Anteflow Gelweave prosthesis after which CPB could be switched to antegrade perfusion. Total cross clamp time was 130 minutes, CPB time was 248 minutes. Intra-operative TOE showed no residual MR, low gradient over the mitral valve and a sufficient aortic valve, without wall motion abnormalities.
The postoperative course was unremarkable. On computed tomography angiography (CTA) of the aorta and peripheral vessels, dissection in the descending aorta was absent, confirming the diagnosis of antegrade TAD (Figure 3). Histology of the dissected ascending aortic wall revealed no evidence for connective tissue disease. Four years later, the patient is still in good clinical condition without recurrence of MR or aortic pathologies.
Discussion
Aortic dissection during cardiac surgery is a rare but potentially catastrophic complication with significant morbidity and mortality. As retrograde perfusion through the femoral vessels is the preferred CPB method in MIMVS, retrograde dissection is a feared complication (5). The most common cause of retrograde dissection is guidewire injury, abdominal aortic atherosclerosis and technical faults during peripheral cannulation (6). However, antegrade TAD has not been described during MIMVS. As the ascending aortic diameter of our patient was not significantly dilated, we were caught by surprise of this complication. As we observed a significantly elongated ascending aorta intra-operatively, this case prompted us to alter our method of preoperative planning in patient work-up for MIMVS. All patients evaluated for a minimally invasive approach now undergo preoperative CTA of the aorta and peripheral vessels to evaluate: aortic dilatation, aortic elongation, abdominal aortic atherosclerosis, peripheral artery disease and tortuosity of the iliofemoral vessels (7). For the definition of aortic elongation, we described aortic lengthening during life (8) and demonstrated ascending aortic length to be increased in patients suffering from TAD (9).
To conclude, TAD is a severe complication which can occur during MIMVS retrogradely, as well as antegradely, regardless of aortic dilatation. Aortic elongation could act as relative contraindication for MIMVS and implementation of CTA in the work-up of MIMVS patients is a valuable preoperative planning method to prevent potentially avoidable complications.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Minimally Invasive Mitral Valve Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.09.10). The series “Minimally Invasive Mitral Valve Surgery” was commissioned by the editorial office without any funding or sponsorship. PSN served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sündermann SH, Sromicki J, Rodriguez Cetina Biefer H, et al. Mitral valve surgery: right lateral minithoracotomy or sternotomy? A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2014;148:1989-1995.e4. [Crossref] [PubMed]
- Hermans SM, Heuts S, Olsthoorn JR, et al. Intraoperative endoscopic view. Asvide 2018;5:800. Available online: http://www.asvide.com/article/view/27615
- Hermans SM, Heuts S, Olsthoorn JR, et al. Postoperative contrast-enhanced computed tomography. Asvide 2018;5:801. Available online: http://www.asvide.com/article/view/27616
- Evangelista A, Flachskampf FA, Erbel R, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr 2010;11:645-58. Erratum in: Eur J Echocardiogr 2011;12:642. [Crossref] [PubMed]
- Murzi M, Cerillo AG, Miceli A, et al. Antegrade and retrograde arterial perfusion strategy in minimally invasive mitral-valve surgery: a propensity score analysis on 1280 patients. Eur J Cardiothorac Surg 2013;43:e167-72. [Crossref] [PubMed]
- Rylski B, Beyersdorf F. Current concepts for minimally invasive mitral valve repair. Heart Lung Vessel 2013;5:207-12. [PubMed]
- Heuts S, Maessen JG, Sardari Nia P. Preoperative planning of left-sided valve surgery with 3D computed tomography reconstruction models: sternotomy or a minimally invasive approach?. Interact Cardiovasc Thorac Surg 2016;22:587-93. [Crossref] [PubMed]
- Adriaans BP, Heuts S, Gerretsen S, et al. Aortic elongation part I: the normal aortic ageing process. Heart 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Heuts S, Adriaans BP, Gerretsen S, et al. Aortic elongation part II: the risk of acute type A aortic dissection. Heart 2018; [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Hermans SMM, Heuts S, Olsthoorn JR, Sardari Nia P. Antegrade type A aortic dissection under endoscopic vision during minimally invasive mitral valve repair: a case report. J Vis Surg 2018;4:211.


