Laryngotracheal resection: perioperative management and surgical technique
Introduction
In the landscape of tracheal surgery, subglottic stenosis still represents a demanding condition because of the technical and functional implications surrounding the peculiar anatomy of the lower larynx (1).
The subglottic airway is a confined non-resilient space of about 2 cm in diameter extending from below the vocal folds to the superior margin of the first tracheal ring. Structurally, it is encircled by the cricoid cartilage, which support the arytenoids (and so the vocal ligaments) on its posterosuperior surface. The recurrent nerves also enter the laryngeal wall at this site just behind the cricothyroid articulations. For these reasons, when performing surgical resection, meticulous care must be taken to spare a shell of the posterior cricoid plate thus preserving structural and nervous entirety of the vocal cords (2).
The etiology of laryngotracheal stenosis is almost always benign. Subglottic airway injuries due to translaryngeal intubation or tracheostomy still represent the main cause. The stenotic scar may develop in response to a mucosal ischaemic damage at the level of the inflated cuff or be secondary to a reiterated mechanical stress by the rigid walls of the endotracheal or tracheostomy tube (3-5). The damage may be extended to the glottis and to the proximal trachea. A postintubation trauma of the posterior subglottic region generally evolves in fibrous thickening of the cricoid plate extended to the interarytenoid space with possible limitation of the vocal cords function. Isolated stenosis of the anterior commissure is rare (3).
Others conditions that can develop in airway obstruction are cervical trauma, repeated caustics inhalation or high-grade irradiation of the neck.
Occasionally, a circumferential fibrotic stenosis of the subglottic space can be diagnosed in patients with no prior history of laryngotracheal diseases. Idiopathic subglottic stenosis is a unique, latently progressive condition affecting almost exclusively middle-aged women. In the past the disease has been associated with miscellaneous collagen vascular diseases, local infections and silent gastroesophageal reflux but, at present, the etiology of this disorder is still unclear (6,7).
Since the basis for a safe and complete surgical excision of subglottic stenosis with primary laryngotracheal anastomosis have been described (2,8), results from large published series have reported excellent and durable success in this setting in view of low morbidity and mortality rates (9-14), thus affirming the role of surgery as the definitive treatment of choice for benign stenosis.
Over the years, through increased technical development, a number of alternative therapeutic options, principally based on mechanical dilation, endoscopic laser ablation and stenting, have been proposed; these procedures, while safe, have shown to bring only momentary benefits in the treatment of subglottic strictures, due to anatomical and technical reasons (15,16). Furthermore, the general practice to repeated bronchoscopic procedures can result in additional airway damage and extension of the diseased tract. Such techniques are therefore mainly employed to expand the airway calibre before resection or to achieve an acceptable palliation in patients not eligible for surgery (10,17,18).
Preoperative assessment
Prior the operation, proper assessment of morphological and functional aspects of each case is essential in order to select those patients who can really benefit from surgery.
An accurate preoperative staging of the extent and characteristics of the damaged segment to be resected helps to determine the best operative approach and anticipate any mobilizing procedures.
Pulmonary function tests evaluation (spirometry, airway resistance, flow-volume loop) may identify whether the dynamic variability of the obstruction and its intra or extrathoracic location (19); nevertheless, due to their low specificity, these procedures are rarely employed in clinical practice.
More morphological details can be obtained through endoscopic and radiologic examination.
Flexible bronchoscopy gains prompt evaluation of the vocal cords function and trophicity, defines the exact position, longitudinal extent and severity of the stricture and identifies the presence of concurrent tracheomalacia (3,15). In critical stenosis, laryngotracheal resection may follows a previous endoscopic dilation procedure; during the preoperative fiberoptic examination of these cases, care must be taken to identify the presence of active mucosal inflammation, as it represents a local contraindication to surgery and needs stabilization of the scar tissue in order to perform the reconstruction on healthy tissues and prevent anastomotic complications (3,12,15).
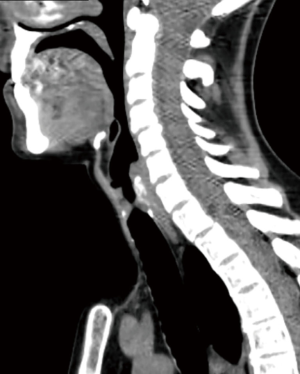
Neck and chest computed tomography (CT) provides a more detailed picture of the tracheal course, its anatomical relationships and wall status (calcifications, cartilaginous fractures) (Figure 1). As feasible, high resolution (1 mm) CT scan with three-dimensional reconstruction is preferable to standard radiological techniques as it perfectly illustrates the longitudinal extension of the stenosis and its relation with the laryngeal structures (14).
Because upper airway stenosis generally occurs after prolonged intubations, it is not uncommon that post-coma patients can be affected by laryngotracheal diseases. In this subgroup of cases, detailed preoperative disclosure targeted to the patient and his family and comprehensive education regarding some critical aspects of the postoperative course have proved fundamental for the final success of surgery (20).
On the functional hand, careful preoperative screening for deglutition abilities and laryngeal competency is mandatory, especially in the case of patients who have neurological impairment. A defect in sphinteric laryngeal function or the presence of swallowing disorders may lead to postoperative chronic aspiration, respiratory complications and pneumonia (21). However, a previous traumatic intubation or tracheotomy may be complicated by a range of laryngeal dysfunctions including abductor failure, arytenoid dislocation, vocal cord paralysis and vocal cord fusion, even when no prior pathology exists. Dysphagia may also be the result of a postintubation injury or may be associated with different neurological diseases. Therefore, a correct identification of each functional disorder and its treatment should always precede surgery.
Perioperative management
Laryngotracheal surgery is a particularly challenging condition for the anesthesiologist. During the operation, an adequate gas exchange must be guaranteed although without affecting visibility of the tracheal lumen, that is essential for the surgeon. Furthermore, the presence of an airway obstruction makes the induction of anesthesia a perous period. Preoperative discussion of the surgical plan and continuous intraoperative dialogue between the surgeon and the anesthesiologist team is crucial to determine the safest intubation technique and to establish a valid and secure ventilation (22).
Airway management is generally effective through conventional endotracheal intubation with a wire-reinforced tube. In most cases of tight stenosis, a rigid small caliber (4–4.5 millimeters) endotracheal tube can be passed through the stricture or placed immediately above it, thus ensuring appropriate ventilation until the trachea is divided (12,20). In patients with pre-existing tracheostomy, thereof is intubated direct and later removed en bloc with the stenotic segment (20). A small caliber sterile tube is then introduced across the operative field in the distal stump. Once the anastomotic sutures have been placed and before the laryngeal and the tracheal ends have to be approached, crossfield intubation is removed and a rhinotracheal (less frequent an orotracheal) tube is advanced beyond the suture line by the anesthesiologist resuming ventilation.
During the time of crossfield intubation a certain degree of local competition with the surgical equipment is unavoidable. To overcome this limitation, some authors have suggested the use of high frequency jet ventilation as good alternative to conventional tube (23,24). With this approach, the injection catheter can be placed at a supraglottic, transglottic or endotracheal level thus minimizing the envelope of the operative field at the time of reconstruction. Jet ventilation is not still risk-free: indeed, several potential complications including barotrauma, bacterial field colonization and intraoperative aspiration have been described (25).
In the event of severe non-stabilized stenosis, preoperative mechanical dilation through rigid devices and/or laser resection may be required. In the current and other authors experiences in subglottic strictures (12,15,17), interventional bronchoscopy has been principally employed to allow stabilization of critical stenosis (and thereby to prevent tracheostomy) or to improve clinical conditions before surgery in high-risk patients. Moreover, it’s our opinion that, whenever possible, it is undeniable to avoid preoperative tracheostomy or endoluminal T-tube placement, since they inevitably result in local chronic inflammation. Furthermore, they have the disadvantages to potentially increase the extent of the damaged airway and to favour bacterial colonization (15). These aspects may have an impact on later surgical indication in those patients who are likely to be reconsidered for surgery because of their improvements in general and/or local conditions. Anyway, in the event of infection at the site of a previous trach stoma, systemic and local antibiotics must be administered and protracted until microbiological sterilization has been achieved (15).
Concerning the airway management at the end of surgery, the preference of some authors, including the current, (12,20,26) is to keep in place a rhinotracheal small uncuffed tube in the awakened patient for about 24 hours to protect the suture line and to facilitate tracheobronchial toilette, and to remove it later under bronchoscopy direct view (Figure 2). Some others used to carry out extubation in the operative room (11,27,28), by restricting transitory persistence of an uncuffed tube only to cases with glottis edema and/or vocal cords stupor. In the event of edema, some surgeons (29) recommend to undertake an interim small tracheostomy or a Montgomery T-tube beneath the anastomosis for as long as remission occur. About that, the present authors suggest a 48–72 hours delay of tube removal, period in which low-dose steroids are administered; with this precaution, definitive early extubation can be obtained in almost all patients without further complications (20).
In general, postoperative oral feeding is reintroduced only a few hours after extubation. To stimulate sensitivity (which is initially poor) the first to be implemented are dense texture foods and thickened fluids with particular emphasis on patients who underwent concomitant cervical release procedures.
The routine use of corticosteroids in the postoperative course is still not universally endorsed. The majority of surgeons (27,28) usually administer short course high-dose steroids only in the event of severe glottis edema but, in the current authors long-term experience (13), systematic administration of low-dosage steroid therapy the next few days after the operation, aimed to relieve glottis edema and hypertrophic repair stenosis, did not result in defects of the healing process.
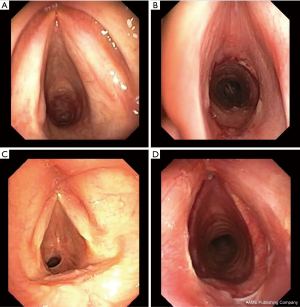
Short- and long-term follow-up is principally based on periodic airway inspections through fiberoptic bronchoscopy (20) (Figure 3).
Surgical technique
The purpose of laryngotracheal reconstructive surgery is to gain an appropriate airway calibre without compromising anatomical and functional integrity of the vocal cords. Because of that (only the anterior portion of the cricoid can be resected) the posterior aspect of the cartilage must be preserved in order to save the laryngeal nerves from surgical injury.
The currently in use surgical technique is still based on the principles described by Pearson and colleagues in 1975 (2). They proposed an innovative modification of previously developed operations that consists in maintaining a shell of the posterior cricoid plate thereby achieving complete resection of the stenotic scar tissues at any level below the vocal cords with respect to recurrent nerves. With these technical arrangements the upper transection line can pass immediately below the vocal cords and, therefore, the thyrotracheal anastomosis can be straight performed just a few millimeters away from the glottis.
The operation is managed under cervical hyperextension through a collar incision, including the trach stoma when present; rarely, a partial median sternotomy may be needed. The trachea is exposed and mobolised from the inferior border of the cricoid cartilage above, to the lower limit of the stenosis below. During this phase, the laryngeal nerves are frequently difficult to identify because they are embedded in the paratracheal scar tissue; maintaining circumferential dissection close to the outer face of the trachea is generally sufficient to avoid nervous damage. First, the airway is transected beneath the stenotic tract and a reinforced small-caliber sterile tube is introduced in the distal trachea through the operative field in order to secure adequate ventilation for the time of reconstruction. Then, the anterior and lateral aspects of the cricoid arch are excised by a section line that starts at the inferior border of the thyroid cartilage anteriorly and continues downwards in an oblique direction on both sides, thus sparing the cricothyroid joints and the posterior plate.
Therefore, the end-to-end anastomosis is carried out by inside-to-outside fashioned interrupted sutures (3-0 absorbable monofilament material) in order to compensate the unavoidable discrepancy in caliber between the subglottic space and the tracheal stump. Stiches are placed from the back (pars membranacea) to the front and left untied. The anesthesiologist tube is then introduced and the cuff is advanced distal to the anastomosis. The cervical hyperextension is removed and laryngeal and tracheal ends are approached applying gentle traction simultaneously on all sutures. Stiches are then tied outside, front to back. By means of these tricks it is possible to obtain a perfect exposure of the operative field while the stiches are placed by preventing traction on the anastomosis as long as all the sutures are tied.
Sometimes, if no tension exists between the two stamps, the current author’s choice is a partial continuous suture (4/0 absorbable material) on the pars membranacea portion of the anastomosis.
In those cases presenting significant mismatch between the stumps, plication of the pars membranacea at the tracheal side may lead further offsetting, as suggested by Pearson (2).
In the event of concurrent thickening of the interarytenoid space as a result of a post intubation glottic injury, total excision of the surplus tissue is crucial to obtain a proper respiratory space and normal adduction of the vocal cords. In such cases, after the scar tissue is removed, the interarytenoid mucosal defect can be filled by a pedicle flap of pars membranacea fashioned from the distal trachea and tailored by resecting one or two cartilaginous tracheal rings, as previously reported by Grillo and colleagues (30).
The major impediments to get a wide respiratory space in patients suffering from subglottic stenosis are the narrow anatomical conformation of the subglottic airway and its stiffness. In last decades, additional modification of the thyrotracheal reconstruction technique have been developed in trying to obtain permanent enlargement of the subglottic space, mostly when the glottis is even involved (31-34).
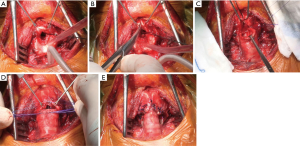
The current authors (35) recently described an original further variation of the technique used to treat severe stenosis extending beyond the glottis level up to the vocal cords, with a special focus on idiopathic stenosis. Once the anterolateral aspect of the cricoid ring is removed, the thyroid cartilage is incised on the midline for about 2 cm along the longitudinal axis reaching the anterior commissure of the larynx (partial laryngofissure) (Figure 4). The edges of the fissure are pulled laterally to increase the airway space thereby facilitating exposure of the posterior region and removal of the exceeding tissue at this level. A running suture is performed for the posterior membranous wall of the anastomosis. Then, 2-mm spaced interrupted stiches are placed on its cartilaginous aspect; the distal trachea is anastomosed directly to the retracted margins of the thyroid cartilage. At the act of the ends approach, the downward traction caused by the knots placed at the apex of the incision and the lateral retraction of the margins of the sectioned thyroid cartilage increase the caliber of the subglottic space, thus reducing discrepancy between the anteroposterior and the laterolateral axes of the proximal trachea. Adequate compensation for caliber discrepancies is provided by the intrinsic elasticity of the cartilaginous tissue.
In all circumstances, at the time of digital dissection an accurate preservation of the tracheal blood supply (provided through a series of small, posterolateral branches) is mandatory to avoid ischaemic postoperative damage and anastomotic dehiscence (3); for this reason, the distal stump should not be isolated circumferentially for more than about 1 cm.
The maximal length of diseased trachea that can be resected has always been a crucial issue. Most subglottic stenosis involves relatively short segments (1–4 centimeter). A temporary limitation of the patients’ cervical hyperextension achieved by placing two strong chin-chest sutures (the “guardian stiches”) at the end of the operation is generally sufficient to minimize tension on the anastomosis (20).
Occasionally, longer segments (often due to reiterated traumatic injuries, repeated endoscopic treatments or tracheostomy) need to be resected. Increasing technical experience in this field has proved that it is possible to carry out an end-to-end reconstruction after removing up to 50% of the total length of the trachea in the adult. Intrinsic elasticity of the tracheal wall, patient’s young age and no history of previous interventional and surgical treatment may positively influence the size of the resection to be performed.
When an extended resection is needed, a tension release procedure must be performed before proceeding with reconstruction. Over the years, various techniques of laryngeal release and hilar release have been proposed (36).
The suprahyoid cervical release, first described by Montgomery in 1974 (37), has proved to be the preferable option for the treatment of laryngotracheal stenosis. Through this manoeuvre, it is possible to gain up to 2 cm of additional mobility of the proximal stump by dividing the insertion of suprahyoid muscles (mylohyoid, geniohyoid and genioglossus) from the superior aspect of the hyoid bone, thus obtaining a significant decrease in tension on the anastomosis without any substantial swallowing sequelae.
Acknowledgments
We wish to thank Dr. Marta Silvi for data management and editorial work.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Federico Rea) for the series “Tracheal surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.10.07). The series “Tracheal surgery” was commissioned by the editorial office without any funding or sponsorship. MI serves as an unpaid editorial board member of Journal of Visualized Surgery from Aug 2017 to Jul 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zeeshan A, Detterbeck F, Hecker E. Laryngotracheal resection and reconstruction. Thorac Surg Clin 2014;24:67-71. [Crossref] [PubMed]
- Pearson FG, Cooper JD, Nelems JM, et al. Primary tracheal anastomosis after resection of the cricoid cartilage with preservation of recurrent laryngeal nerves. J Thorac Cardiovasc Surg 1975;70:806-16. [PubMed]
- Pearson FG, Andrews MJ. Detection and management of tracheal stenosis following cuffed tube tracheostomy. Ann Thorac Surg 1971;12:359-74. [Crossref] [PubMed]
- Colice GL, Stukel TA, Dain B. Laryngeal complications of prolonged intubation. Chest 1989;96:877-84. [Crossref] [PubMed]
- Cooper JD. Tracheal injuries complicating prolonged intubation and tracheostomy. Thorac Surg Clin 2018;28:139-44. [Crossref] [PubMed]
- Grillo HC, Mark EJ, Mathisen DJ, et al. Idiopathic laryngotracheal stenosis and its management. Ann Thorac Surg 1993;56:80-7. [Crossref] [PubMed]
- Donahoe L, Keshavjee S. Contemporary management of idiopathic laryngotracheal stenosis. Thorac Surg Clin 2018;28:167-75. [Crossref] [PubMed]
- Gerwat J, Bryce DP. The management of subglottic laryngeal stenosis by resection and direct anastomosis. Laryngoscope 1974;84:940-57. [Crossref] [PubMed]
- Bisson A, Bonnette P, el Kadi NB, et al. Tracheal sleeve resection for iatrogenic stenoses (subglottic laryngeal and tracheal). J Thorac Cardiovasc Surg 1992;104:882-7. [PubMed]
- Couraud L, Jougon J, Velly JF. Surgical treatment of nontumoral stenosis of the upper airway. Ann Thorac Surg 1995;60:250-9. [Crossref] [PubMed]
- Macchiarini P, Verhoye J, Chapelier A, et al. Partial cricoidectomy with primary thyrotracheal anastomosis for postintubation subglottic stenosis. J Thorac Cardiovasc Surg 2001;121:68. [Crossref] [PubMed]
- Marulli G, Rizzardi G, Bortolotti L, et al. Single-staged laryngotracheal resection and reconstruction for benign strictures in adults. Interact Cardiovasc Thorac Surg 2008;7:227-30; discussion 230. [Crossref] [PubMed]
- D'Andrilli A, Maurizi G, Andreetti C, et al. Long-term results of laryngotracheal resection for benign stenosis from a series of 109 consecutive patients. Eur J Cardiothorac Surg 2016;50:105-9. [Crossref] [PubMed]
- Axtell AL, Mathisen DJ. Idiopathic subglottic stenosis: techniques and results. Ann Cardiothorac Surg 2018;7:299-305. [Crossref] [PubMed]
- Ciccone AM, De Giacomo T, Venuta F, et al. Operative and non-operative treatment of benign subglottic laryngotracheal stenosis. Eur J Cardiothorac Surg 2004;26:818-22. [Crossref] [PubMed]
- Melkane AE, Matar NE, Haddad AC, et al. Management of postintubation tracheal stenosis: appropriate indications make outcome differences. Respiration 2010;79:395-401. [Crossref] [PubMed]
- Wood DE. Bronchoscopic preparation for airway resection. Chest Surg Clin N Am 2001;11:735-48. [PubMed]
- Charokopos N, Foroulis CN, Rouska E, et al. The management of post-intubation tracheal stenoses with self-expandable stents: early and long-term results in 11 cases. Eur J Cardiothorac Surg 2011;40:919-24. [PubMed]
- Gamsu G, Borson DB, Webb WR, et al. Structure and function in tracheal stenosis. Am Rev Respir Dis 1980;121:519-31. [Crossref] [PubMed]
- D'Andrilli A, Ciccone AM, Venuta F, et al. Long-term results of laryngotracheal resection for benign stenosis. Eur J Cardiothorac Surg 2008;33:440-3. [Crossref] [PubMed]
- Goldsmith T. Evaluation and treatment of swallowing disorders following endotracheal intubation and tracheostomy. Int Anesthesiol Clin 2000;38:219-42. [Crossref] [PubMed]
- Roman PEF, Battafarano RJ, Grigore AM. Anesthesia for tracheal reconstruction and transplantation. Curr Opin Anaesthesiol 2013;26:1-5. [Crossref] [PubMed]
- Adelsmayr E, Keller C, Erd G, et al. The laryngeal mask and high-frequency jet ventilation for resection of high tracheal stenosis. Anesth Analg 1998;86:907-8. [Crossref] [PubMed]
- Biro P, Hegi TR, Weder W, et al. Laryngeal mask airway and high-frequency jet ventilation for the resection of a high-grade upper tracheal stenosis. J Clin Anesth 2001;13:141-3. [Crossref] [PubMed]
- Jaquet Y, Monnier P, Van Melle G, et al. Complications of different ventilation strategies in endoscopic laryngeal surgery: a 10-year review. Anesthesiology 2006;104:52-9. [Crossref] [PubMed]
- Couraud L, Chevalier P, Bruneteau A, et al. Treatment of tracheal stenosis after tracheotomy. Therapeutic indications, preparation for the intervention. Apropos of 9 resections in 15 cases of acute respiratory insufficiency. Ann Chir Thorac Cardiovasc 1969;8:791-7. [PubMed]
- Ashiku SK, Kuzucu A, Grillo HC, et al. Idiopathic laryngotracheal stenosis: effective definitive treatment with laryngotracheal resection. J Thorac Cardiovasc Surg 2004;127:99-107. [Crossref] [PubMed]
- Wright CD, Grillo HC, Wain JC, et al. Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg 2004;128:731-9. [Crossref] [PubMed]
- Morcillo A, Wins R, Gómez-Caro A, et al. Single-staged laryngotracheal reconstruction for idiopathic tracheal stenosis. Ann Thorac Surg 2013;95:433-9; discussion 439. [Crossref] [PubMed]
- Grillo HC. Primary reconstruction of airway after resection of subglottic laryngeal and upper tracheal stenosis. Ann Thorac Surg 1982;33:3-18. [Crossref] [PubMed]
- McCaffrey TV. Management of subglottic stenosis in the adult. Ann Otol Rhinol Laryngol 1991;100:90-4. [Crossref] [PubMed]
- Maddaus MA, Toth JL, Gullane PJ, et al. Subglottic tracheal resection and synchronous laryngeal reconstruction. J Thorac Cardiovasc Surg 1992;104:1443-50. [PubMed]
- Couraud L, Jougon JB, Ballester M. Techniques of management of subglottic stenoses with glottic and supraglottic problems. Chest Surg Clin N Am 1996;6:791-809. [PubMed]
- Terra RM, Minamoto H, Carneiro F, et al. Laryngeal split and rib cartilage interpositional grafting: treatment option for glottic/subglottic stenosis in adults. J Thorac Cardiovasc Surg 2009;137:818-23. [Crossref] [PubMed]
- Ciccone AM, Vanni C, Maurizi G, et al. A novel technique for laryngotracheal reconstruction for idiopathic subglottic stenosis. Ann Thorac Surg 2016;102:e469-71. [Crossref] [PubMed]
- Heitmiller RF. Tracheal release maneuvers. Chest Surg Clin N Am 1996;6:675-82. [PubMed]
- Montgomery WW. Suprahyoid release for tracheal anastomosis. Arch Otolaryngol 1974;99:255-60. [Crossref] [PubMed]
Cite this article as: Vanni C, Poggi C, D’Andrilli A, Ciccone AM, Ibrahim M, Andreetti C, Menna C, Massullo D, Venuta F, Rendina EA, Maurizi G. Laryngotracheal resection: perioperative management and surgical technique. J Vis Surg 2018;4:216.