
Management of intraoperative bleeding during thoracoscopic pulmonary resection in Japan
IntroductionOther Section
- Introduction
- How to manage intraoperative bleeding during thoracoscopic surgery
- Summary
- Acknowledgments
- Footnote
- References
Total thoracoscopic pulmonary resection is used worldwide, partly because of the good results that have been reported in recent years. Many authors have reported that the thoracoscopic approach is less invasive and provides greater benefits, such as more rapid postoperative recovery and shorter hospitalization, than thoracotomy approach although a prospective randomized trial to compare open thoracotomy and thoracoscopic lobectomy has yet to be conducted (1,2). In addition, the prognosis of patients who undergo surgery via the thoracoscopic approach may not be inferior to that of patients who undergo thoracotomy for early-stage lung cancer. This would make the thoracoscopy the preferred approach for early-stage lung cancer (2).
In thoracoscopic surgery, to ensure the safety of the operation and minimize the rate of conversion to thoracotomy, it is important to emphasize appropriate troubleshooting, particularly for intraoperative bleeding, because inappropriate management of significant bleeding can lead to catastrophic and life-threatening consequences. In addition, the management of vascular injury is considered to be much more complex during thoracoscopic surgery than during thoracotomy. This may be due to the difficulty achieving a vascular clamp as well as in the subsequent suturing of the injured site in intrathoracic vessels using a monitor during the thoracoscopic approach. Therefore, a simpler technique may be needed to achieve appropriate hemostasis in the thoracoscopic approach.
Various reports have described the management of intraoperative bleeding during thoracoscopic pulmonary resection. However, the strategy for hemostasis has differed among studies because the thoracoscopic approach is not a uniform procedure; thus, port positions, number of ports, length of incision, use/no use of an access port, and visualization of the surgical field either directly or via a monitor have varied (3-5). This has made it difficult to standardize the troubleshooting of issues such as intraoperative bleeding during thoracoscopic surgery.
Only a few studies have focused on the surgical techniques used to manage massive intraoperative bleeding during thoracoscopic surgery, while many have described perioperative results, complications, and prognoses (6-13). To the best of our knowledge, only three studies reported in the English language have focused on troubleshooting for intraoperative bleeding during thoracoscopic surgery in Japan (11-13).
In this study, we reviewed the surgical techniques used to achieve hemostasis during thoracoscopic surgery and investigated the appropriate management of intraoperative bleeding.
How to manage intraoperative bleeding during thoracoscopic surgeryOther Section
- Introduction
- How to manage intraoperative bleeding during thoracoscopic surgery
- Summary
- Acknowledgments
- Footnote
- References
In our thoracoscopic surgery, a monitor was placed above the head of the patients and all procedures were carried out through the thoracoscopic vision. We used 5- or 10-mm flexible-type thoracoscope. The operating surgeon stood ventral to the patient, while the assistant surgeon in a dorsal position. The access incision (initially 3 cm in length) was in the 4th intercostal space on the anterior axillary line. While a port for a thoracoscope was placed in the 6th intercostal space on the posterior axillary line, two additional ports were placed in the 6th intercostal space on the anterior axillary line and inferior angle of scapula. These three ports except for the thoracoscopic port were covered with a size XXS wound retractor (Alexis Wound Retractor; Applied Medical, Rancho Santa Margarita, CA, USA). Large vessels and bronchi were usually divided by an endostapler. Hemostasis of small-caliber vessels was performed by ligation or using an electrocoagulator or a combination of both. On completion of the pulmonary resection, the specimen was placed into an endovascular bag and retrieved through the access port. If necessary, the incision was enlarged, depending on the specimen size. A rib-spreader was never used at any point during the operation. Among patients with primary lung cancer, systemic lymphadenectomy was performed for those who underwent lobectomy, and sampling of hilar lymph nodes was performed for those who underwent segmentectomy. No lymphadenectomy was performed for patients with metastatic lung cancer or benign disease. Finally, a drainage tube was placed in the thorax through the 6th intercostal port on the anterior axillary line.
We have established a novel algorithm for managing significant intraoperative bleeding (13). Significant bleeding was defined as the bleeding which required compression for longer than 30 seconds to get hemostasis. Using that algorithm, the bleeding site of an intrathoracic vessel is treated by compression with lung parenchyma or a cotton stick. In addition, thrombostatic sealants, TachoSil (CSL Behring, Japan), are usually attached to the bleeding site to avoid intraoperative or postoperative re-bleeding. The sealant is initially cut into a 7×7 mm square and then attached to the top of a cotton stick using medical jelly. Then it is introduced into the thorax via a port and applied to the bleeding site. A second or third piece of sealant is added if necessary. This technique is easier to perform than suturing the injured vessels. Therefore, it is suitable for achieving hemostasis during thoracoscopic surgery.
The wall of the pulmonary artery is thin and fragile compared to other intrathoracic vessels. Therefore, it is often injured, and can lead to catastrophic bleeding if not treated appropriately (6,7,11,13). In view of this, attachment of a thrombostatic sealant is a simple and suitable method for achieving homeostasis in thoracoscopic surgery. In our previous study, 44.4% of significant intraoperative bleeding during thoracoscopic surgery was caused by injury of pulmonary arterial branches, and most of them were treated by compression of the adjacent lung and/or attachment of thrombostatic sealant (13).
Following our algorithm, the bleeding site is usually compressed using the adjacent lung or a cotton stick to control bleeding prior to the attachment of the thrombostatic sealant (13). This compression technique is frequently utilized in Japan to temporally control bleeding (11,14). Moreover, Miyamoto et al. (14) recently reported the useful technique of “lung folding”. “Lung folding technique” is very similar to compression of the lung; however, there is a slight difference between the two techniques. Miyamoto et al. insisted that the adjacent lung should be folded. Doing so traps the bleeding site between the adjacent lung and the hilar structure, which can provide more stable compression than traditional compression of the lung alone. We have modified our algorithm to include this step (Figure 1).
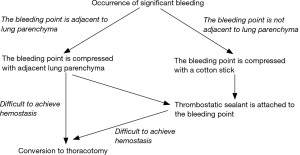
Injury to other vessels such as the pulmonary vein, azygos vein, superior vena cava, subclavian artery or vein, and bronchial artery can also be catastrophic. While thrombostatic sealant is again mainly used to control bleeding from low-pressure vessels including the pulmonary vein, azygos vein or superior vena cava, high-pressure vessels such as systemic arteries may not be amenable to repair using sealant. Previously, Yamashita et al. (11) reported that application of a sealant to a subclavian arterial injury resulted in a pseudoaneurysm. They recommended using thrombostatic sealants for low-pressure vessels only. Therefore, clipping or ligation might be a better way to achieve hemostasis when these high-pressure vessels were injured although we have experienced successful hemostasis of bleeding from bronchial arterial injury.
Along with this report, we include videos that illustrate both the attachment of a thrombostatic sealant and compression of the injury site by the adjacent lung to achieve hemostasis during thoracoscopic surgery (Figures 2,3).


SummaryOther Section
- Introduction
- How to manage intraoperative bleeding during thoracoscopic surgery
- Summary
- Acknowledgments
- Footnote
- References
In Japan, attachment of a thrombostatic sealant and compression of an injury site using the adjacent lung to achieve hemostasis during thoracoscopic surgery are frequently utilized; these techniques are considered of great importance in reducing the conversion rate to thoracotomy. However, all of the studies that describe troubleshooting for intraoperative bleeding have suggested that surgeons should not hesitate to convert from thoracoscopic surgery to thoracotomy when difficulty arises in controlling intraoperative bleeding because such bleeding, particularly in the case of a pulmonary arterial injury, might be catastrophic. Therefore, surgeons should become skilled in the fast and smooth conversion to thoracotomy as well as in providing appropriate hemostasis in thoracoscopic surgery.
AcknowledgmentsOther Section
- Introduction
- How to manage intraoperative bleeding during thoracoscopic surgery
- Summary
- Acknowledgments
- Footnote
- References
Funding: None.
FootnoteOther Section
- Introduction
- How to manage intraoperative bleeding during thoracoscopic surgery
- Summary
- Acknowledgments
- Footnote
- References
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Emergency Response to Intraoperative Bleeding”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.10.09). The series “Emergency Response to Intraoperative Bleeding” was commissioned by the editorial office without any funding or sponsorship. KS served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- How to manage intraoperative bleeding during thoracoscopic surgery
- Summary
- Acknowledgments
- Footnote
- References
- Jeon JH, Kang CH, Kim HS, et al. Video-assisted thoracoscopic lobectomy in non-small-cell lung cancer patients with chronic obstructive pulmonary disease is associated with lower pulmonary complications than open lobectomy: a propensity score-matched analysis. Eur J Cardiothorac Surg 2014;45:640-5. [Crossref] [PubMed]
- Stephens N, Rice D, Correa A, et al. Thoracoscopic lobectomy is associated with improved short-term and equivalent oncological outcomes compared with open lobectomy for clinical Stage I non-small-cell lung cancer: a propensity-matched analysis of 963 cases. Eur J Cardiothorac Surg 2014;46:607-13. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S, et al. Locoregional control of thoracoscopic lobectomy with selective lymphadenectomy for lung cancer. Ann Thorac Surg 2010;90:235-9. [Crossref] [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Hybrid surgical approach of video-assisted minithoracotomy for lung cancer: significance of direct visualization on quality of surgery. Chest 2005;128:2696-701. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Flores RM, Ihekweazu U, Dycoco J, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg 2011;142:1412-7. [Crossref] [PubMed]
- Fournel L, Zaimi R, Grigoroiu M, et al. Totally thoracoscopic major pulmonary resections: an analysis of perioperative complications. Ann Thorac Surg 2014;97:419-24. [Crossref] [PubMed]
- Jones RO, Casali G, Walker WS. Does failed video-assisted lobectomy for lung cancer prejudice immediate and long-term outcomes?. Ann Thorac Surg 2008;86:235-9. [Crossref] [PubMed]
- Liang C, Wen H, Guo Y, et al. Severe intraoperative complications during VATS Lobectomy compared with thoracotomy lobectomy for early stage non-small cell lung cancer. J Thorac Dis 2013;5:513-7. [PubMed]
- Mei J, Pu Q, Liao H, et al. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary resection without conversion to thoracotomy. Surg Endosc 2013;27:530-7. [Crossref] [PubMed]
- Yamashita S, Tokuishi K, Moroga T, et al. Totally thoracoscopic surgery and troubleshooting for bleeding in non-small cell lung cancer. Ann Thorac Surg 2013;95:994-9. [Crossref] [PubMed]
- Sawada S, Komori E, Yamashita M. Evaluation of video-assisted thoracoscopic surgery lobectomy requiring emergency conversion to thoracotomy. Eur J Cardiothorac Surg 2009;36:487-90. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Ibe T, et al. Troubleshooting for bleeding in thoracoscopic anatomic pulmonary resection. Asian Cardiovasc Thorac Ann 2017;25:35-40. [Crossref] [PubMed]
- Miyamoto Y. Thoracoscopic management of pulmonary artery bleeding. Nihon Geka Gakkai Zasshi 2017;118:71-3. [PubMed]
- Igai H, Kamiyoshihara M, Yoshikawa R, et al. The procedure for the attachment of a thrombostatic sealant to the bleeding site. Asvide 2018;5:831. Available online: http://www.asvide.com/article/view/27986
- Igai H, Kamiyoshihara M, Yoshikawa R, et al. The procedure for compressing the bleeding site using the adjacent lung. Asvide 2018;5:832. Available online: http://www.asvide.com/article/view/27987
Cite this article as: Igai H, Kamiyoshihara M, Yoshikawa R, Ohsawa F, Yazawa T, Shimizu K. Management of intraoperative bleeding during thoracoscopic pulmonary resection in Japan. J Vis Surg 2018;4:225.



