Video-assisted thoracoscopic surgery in bacterial empyema thoracic result from developing country based on Thailand experience
Introduction
Empyema thoracic is a common disease in worldwide (1-6). An incidence of this disease has been gradually increased every year (7-12). In 1962, the American Thoracic Society was described the 3 phases of empyema as exudative (stage I), fibrinopurulent (stage II), and organizing (stage III) (13,14). An exudative phase which can be treated by intercostal chest drainage and appropriate antibiotic coverage (10). However, failure in medical treatment of this stage will require a surgical approach (15). Patients with stage II and III usually require a surgical intervention due to thick fibrin peel which deposits at visceral pleural surface causing entrapment of lung or loculated pleural effusion. Gold standard surgical approach for these stages is a conventional open thoracotomy (OT), however over past decade video-assisted thoracoscopic surgery (VATS) has increasing popularity and become a safe procedure for thoracic operations with showing a benefit of reducing pain, and morbidity as well as shorter length of hospital stay (16). Despite of those benefit, VATS also had drawbacks such as increased operative time, increased cost, steeper learning curve, and incomplete treatment. For role of VATS in empyema thoracic is still questionable due to technical demanding and time consuming (10,17,18).
This current study, we review the results of surgical outcomes in bacterial empyema thoracis (BET) comparing between VATS and OT approach from our country experience.
Methods
Cardiothoracic surgery units from six hospitals; Vajira Hospital, Ramathibodi Hospital, Thammasat Hospital, Chiangmai University Hospital, Siriraj Hospital and Bangkok Hospital in Thailand were collaborated in this study. This study was reviewed and approved by local research ethics committee in each hospital. All patients were confirmed to diagnose BET by diagnostic thoracentesis and radiologic imaging (computed tomography). Patients who have thoracic malignancy causing empyema thoracis, confirmed tuberculous empyema, and empyema thoracis associated with procedures were excluded from this study.
Preoperative management of BET in all hospitals was treated according to the British Thoracic Society guidelines (15). All patients who received decortication or drainage procedure either OT approach or VATS approach were depended surgeon preference. VATS approach was recommended as a first line for treatment in empyema thoracic. For those patients who had previous history of thoracotomy or pleurodesis or empyema thoracic or fibrothorax and inability for tolerate single lung ventilation were relative contraindication for VATS approach.
All patients were given appropriate intravenous antibiotics (broad-spectrum) based on the result from pus culture. No fibrinolytic therapy was used in any patient.
Patient demographics and clinical parameters were retrospectively reviewed from medical recording system included age and sex, Charlson comorbidity index, smoking status, stage of empyema, laterality, cause of empyema thoracic, underlying tuberculosis, symptoms (fever, cough, chest pain and dyspnea), antibiotic prior surgery, duration of antibiotic administration prior surgery and operation types. Chest radiographs and computed tomography were obtained in all patients.
The intraoperative parameters and postoperative outcomes were monitored to assess the progress and outcomes of patients included complete decortication, immediate extubating, duration of intensive care unit (ICU) stay, duration of tube drainage, duration of hospital stay, blood loss in operative, blood transfusion, perioperative complications, and rate of lung expansion.
The primary outcomes were postoperative complications included re-intubation, prolonged air leak, atelectasis, pneumonia, tracheostomy, wound infection, sepsis shock and 30-day mortality.
Surgical technique
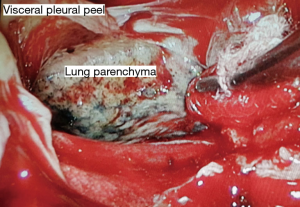
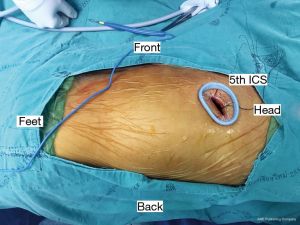
All procedures were performed with general anesthesia using lung isolation technique with a double-lumen endotracheal tube or bronchial blocker. Surgical operations were performed by board-certified cardiovascular thoracic surgeons. The principle of both surgical approaches is to drain all loculated fluid collections and enable full lung re-expansion by removal of pleural peel from visceral pleura known as decortication. For OT approach, standard technique of posterolateral thoracotomy, lateral thoracotomy or muscle sparing thoracotomy with rib spreading was used. For VATS approach, the number of ports was depended on surgeon techniques in each hospital. For my approach, we routinely performed 2 port VATS. First, 4 cm incision was incised at 5th ICS in anterior axillary line for utility port (Figure 1) and then the operator’s index finger was introduced into the thoracic cavity for digital exploration which will assess was found to be helpful in assessing the stage of the empyema and dissection circumferentially. During cautiously blind dissection, we usually ventilate both lung using low tidal volume to prevent false dissected plane causing parenchymal lung injury. Camera port was inserted near utility port. All fibrin, loculated and septate of effusion were removed by suction under endoscopic control. Then, lung parenchyma should be dissected completely free from the parietal pleura, diaphragmatic surface and mediastinum pleura. Decortication (Figure 2) was done with dissector holding a peanut, Maryland gasper and suction. After the procedure, lung should be fully expanded. If inadequate drainage was suspected or uneventful was occur, conventional thoracotomy will be performed. After completion of the procedure, two large-bore chest tubes (28- or 32-French) were inserted through the ports and placed under endoscopic vision in the costodiaphragmatic sulcus and posterior chest wall, respectively, to drain fluid and air leaks after the operation (Figure 3). Extubation was performed immediately after operation, in the recovery room or in the ICU depending on the consideration of the anesthesiologists. Chest X-ray was done within 8–12 hours postoperatively. Routine postoperative care included adequate pain control, pulmonary toilet exercise and early mobilization was performed. Chest drain was removed if the content drainage was clear (no fresh blood or pus), less than 200 mL/day and no air leakage. All patients were followed and evaluated the clinical symptoms with chest X-ray at the cardiovascular thoracic clinic 2 weeks after discharged.

Statistical analysis
Categorical variables were presented as frequency and percent. Continuous variables were presented as mean and standard deviation or median and interquartile range depending on data distribution. Fisher’s exact test and Student t-test or Wilcoxon’s rank-sum were used to compare categorical variables and continuous variable respectively. Two-step analysis was performed. Logistic regression was used to calculate a propensity score (PS), which evaluates confounding by indication. The variables included in the model for PS were age, sex, Charlson comorbidity index, smoking status, stage of disease, laterality, duration of symptom prior diagnosis, operative procedures, and surgeon preference (institutes); the score was then divided into quintiles, called PS-groups. A multilevel model stratified by PS-groups was used for comparing the postoperative outcomes between groups. A multicollinearity test was done. A P value <0.05 was considered to be statistically significant. All statistically analysis was performed using STATA program version 15.1 (STATA Corp, CS, TX, USA).
Results
A total of 300 patients diagnosed with BET were included in this study. Ninety-eight (32%) patients were undergoing VATs approach whereas 202 patients (68%) were undergoing OT approach. Two-third of patients presented with fever (63.6%), cough (63.3%), and dyspnea (61.3%). Most of patients had stage II or III of disease. Most common cause of BET was pneumonia (76%) followed by lung abscess (10.7%) and trauma (5.7%). Only 28.7% of pus culture was positive culture for bacteria before operation.
Patient characteristics comparing between two groups are shown in Table 1. There was no significant difference in terms of age, median time of duration of antibiotic administration before surgery, underlying tuberculosis (TB) infection, and drainage procedure. The VATS group had more patients with female, higher Charlson comorbidity index, higher active smoker, more diagnosed with stage II of disease, shorter duration of symptoms prior to diagnosis, lower proportion of positive pus culture before surgery, and lower underwent decortication than in the thoracotomy group. The PS (probability of receiving certain treatment) showed a statistically significant difference, therefore the overall preoperative patient characteristics in each approach group were different.
Table 1
| Variables | Total (n=300) | VATS (n=98) | Thoracotomy (n=202) | P value |
|---|---|---|---|---|
| Age (year), mean (SD) | 50.32 (18.23) | 52.20 (16.02) | 49.41 (19.19) | 0.213 |
| Gender, n (%) | 0.008 | |||
| Male | 231 (77.00) | 66 (67.35) | 165 (81.68) | |
| Female | 69 (23.00) | 32 (32.65) | 37 (18.32) | |
| CCI index, median (IQR) | 1 [1–3] | 2 [1–3] | 1 [1–2] | 0.020 |
| Smoking status, n (%) | <0.001 | |||
| Non-smoker | 140 (46.67) | 51 (51.52) | 89 (44.06) | |
| Ex-smoker | 103 (34.33) | 18 (18.18) | 85 (42.08) | |
| Active smoke | 57 (19.00) | 30 (30.30) | 28 (13.86) | |
| Stage of empyema (%) | 0.002 | |||
| 1 | 2 (0.67) | 0 | 2 (0.99) | |
| 2 | 56 (18.67) | 29 (29.59) | 27 (13.37) | |
| 3 | 242 (80.67) | 69 (70.41) | 173 (85.64) | |
| Laterality, n (%) | 0.081 | |||
| Rt | 173 (57.67) | 64 (64.31) | 109 (53.96) | |
| Lt | 127 (42.33) | 34 (35.69) | 93 (46.04) | |
| Presentation, n (%) | ||||
| Fever | 191 (63.67) | 72 (73.47) | 119 (58.91) | 0.015 |
| Cough | 190 (63.33) | 70 (71.43) | 120 (59.41) | 0.055 |
| Dyspnea | 184 (61.33) | 59 (60.20) | 125 (61.88) | 0.801 |
| Chest pain | 122 (40.67) | 56 (57.14) | 66 (32.67) | <0.001 |
| Duration of symptoms prior to diagnosis (day), median (IQR) | 14 [6–30] | 7 [3–28] | 14 [7–30] | 0.007 |
| ATB administration before surgery, n (%) | 263 (87.67) | 77 (78.57) | 186 (92.08) | 0.001 |
| Duration of ATB administration before surgery (day), median (IQR) | 10.5 [4–19] | 12 [5–17.50] | 10 [4–19.50] | 0.630 |
| Cause of Empyema thoracic, n (%) | 0.183 | |||
| Pneumonia | 228 (76.00) | 80 (81.63) | 148 (73.27) | |
| Lung abscess | 32 (10.67) | 10 (10.20) | 22 (10.89) | |
| Trauma | 17 (5.67) | 5 (5.10) | 12 (5.94) | |
| Other | 23 (7.67) | 3 (3.06) | 20 (9.90) | |
| Underlying TB, n (%) | 26 (8.67) | 7 (7.14) | 19 (9.41) | 0.663 |
| Pus culture positive before operation, n (%) | 86 (28.67) | 19 (19.39) | 67 (33.17) | 0.014 |
| Operation, n (%) | ||||
| Drain | 92 (30.67) | 36 (36.73) | 56 (27.72) | 0.142 |
| Decortication | 251 (83.67) | 74 (75.51) | 177 (87.62) | 0.012 |
| Propensity score, median (IQR) | 0.13 (0.07–0.82) | 0.90 (0.56–0.95) | 0.09 (0.06–0.15) | <0.001 |
VATS, video-assisted thoracoscopic surgery; CCI, Charlson comorbidity index; ATB, antibiotic; IQR, interquartile range; TB, tuberculosis; SD, standard deviation; ATB, antibiotic.
Postoperative outcomes comparing between two groups are shown in Table 2. There was no significant difference in terms of complete decortication (92.9% in VATS vs. 96.04% in thoracotomy group), operative time, blood loss, postoperative pneumonia, atelectasis, re-operation, tracheostomy, septic shock, composite postoperative complication, ICU need, duration of ventilator need, fully expanded lung, recurrence of disease, length of hospital stays, and in-hospital mortality. There was also no significant difference in mortality rate (9.18% in VATS and 7.43% in thoracotomy group). Most common cause of death in both groups were related to septic shock. The VATS group had patients with lesser blood transfusion; lower proportion of peri-operative complications (hypotension and bleeding), re-intubation, wound infection, and ventilator need; higher proportion of immediate extubation; shorter length of ICU stay, and length of retaining chest drain than in thoracotomy group. Re-operation insulted from prolong air leak and inadequate drainage. However, because of difference in preoperative patient characteristics between two groups, postoperative outcomes comparing between groups was analyzed using a multilevel model stratified by PS (Table 3) and showed no significant differences between groups in terms of blood transfusion, composite postoperative complications and length of retaining chest tube drainage. Perioperative complications, re-intubation, wound infection, and length of ICU stay were significant less in the VATS group.
Table 2
| Variables | Total (n=300) | VATS (n=98) | Thoracotomy (n=202) | P value |
|---|---|---|---|---|
| Complete decortications, n (%) | 285 (95.00) | 91 (92.86) | 194 (96.04) | 0.264 |
| Operative time (mins), median (IQR) | 100 (73.50, 142) | 105 (75, 139) | 95 (65, 147) | 0.176 |
| Blood loss (mL), median (IQR) | 300 (150, 500) | 300 (200, 600) | 300 (100, 500) | 0.089 |
| Blood transfusion, (%) | 155 (51.67) | 35 (35.71) | 120 (59.41) | <0.001 |
| Peri-operative complications (%) | 36 (12.00) | 4 (4.08) | 32 (15.84) | 0.002 |
| Immediate extubating (%) | 197 (65.67) | 75 (76.53) | 122 (60.40) | 0.006 |
| Complication, n (%) | 79 (26.33) | 19 (19.39) | 60 (29.70) | 0.069 |
| Re-intubation | 24 (8.00) | 3 (3.06) | 21 (10.40) | 0.039 |
| Pneumonia | 22 (7.33) | 4 (4.08) | 18 (8.91) | 0.161 |
| Atelectasis | 11 (3.67) | 2 (2.04) | 9 (4.46) | 0.513 |
| Re-operation | 29 (9.67) | 8 (8.16) | 21 (10.40) | 0.678 |
| Tracheostomy | 14 (4.67) | 3 (3.06) | 11 (5.45) | 0.560 |
| Wound infection | 23 (7.67) | 1 (1.02) | 22 (10.89) | 0.002 |
| Septic shock | 25 (8.33) | 6 (6.12) | 19 (9.41) | 0.382 |
| ICU need, n (%) | 145 (48.33) | 48 (48.98) | 97 (48.02) | 0.902 |
| Length of ICU stay (days), median (IQR) | 3 (2, 8) | 1 (1, 4) | 4 (2, 11) | <0.001 |
| Ventilation dependence, n (%) | 105 (35.00) | 24 (24.49) | 81 (40.10) | 0.010 |
| Duration of ventilator need (days), median (IQR) | 4 (2, 8) | 4 (1, 6) | 4 (2, 9) | 0.382 |
| Death, n (%) | 24 (8.00) | 9 (9.18) | 15 (7.43) | 0.652 |
| Discharge status, n (%) | 1.000 | |||
| Not improve | 28 (9.33) | 9 (9.18) | 19 (9.41) | |
| Improve | 272 (90.67) | 89 (90.82) | 183 (90.59) | |
| Lung expand, n (%) | 0.846 | |||
| Partial expand | 34 (11.33) | 10 (10.20) | 24 (11.88) | |
| Full expand | 266 (88.67) | 88 (89.90) | 178 (88.12) | |
| Recurrent, n (%) | 0.169 | |||
| No | 284 (94.67) | 90(91.84) | 194 (96.04) | |
| Yes | 16 (5.33) | 8(8.16) | 8 (3.96) | |
| Hospital stay (day), median (IQR) | 10 (7, 17) | 9 (6, 17) | 10 (7, 18) | 0.191 |
| Chest drain duration (day), median (IQR) | 6 (4, 9) | 5 (3, 8) | 6 (4, 10) | 0.009 |
VATS, video-assisted thoracoscopic surgery; IQR, inter quartile range; ICU, intensive care unit.
Table 3
| Variables | Risk ratio | Mean difference | 95% CI | P value |
|---|---|---|---|---|
| Blood transfusion, (%) | 0.71 | 0.26–1.95 | 0.503 | |
| Perioperative complications | 0.26 | 0.09–0.71 | 0.009 | |
| Re-intubation | 0.29 | 0.09–0.98 | 0.048 | |
| Wound infection | 0.09 | 0.01–0.71 | 0.022 | |
| Composite peri-operative complication* | 0.99 | 0.45–2.15 | 0.977 | |
| Duration of ICU stay, (days) | −4.36 | −8.55 to −0.18 | 0.041 | |
| Ventilator needed, (%) | 0.61 | 0.38–0.96 | 0.034 | |
| Retaining chest drain duration (days) | −3.74 | −12.23 to 4.74 | 0.388 |
*, composite peri-operative complications include re-intubation, pneumonia, atelectasis, reoperation, tracheostomy, wound infection, and septic shock. VATS, video-assisted thoracoscopic surgery; ICU, intensive care unit; CI, confidence interval.
Discussion
Empyema thoracic is a pleural cavity infection. Most common cause is parapneumonic effusion which usually develop in 50–70% of pre-existing pneumonia patients, and 20% percent of those effusion will turn into purulent empyema thoracis (20,21). The effect of empyema thoracic could cause 10–40% of morbidity and mortality rate (3,4,22,23). Currently, a standard treatment for BET is still a conventional OT decortication; however, this traditional thoracotomy is associated with postoperative pain and morbidity (24). Recently in 2015, based on expert consensus statement, the European Association for Cardio-Thoracic Surgery has recommended VATS-approach is a primary surgical management for BET (24). The advantages of VATS approach are less postoperative pain control, shorter length of stay, less blood loss, less respiratory compromise, and reduction in postoperative complications as well as 30-day mortality in VATS approach (25-28).
Our study found that perioperative complications, re-intubation, wound infection, and length of ICU stay were significant less in the VATS group. Success rate of complete decortication can perform via VATS approach comparable with OT and can achieve fully expanded lung.
Tong and his colleague reported a large series comparing between VATS and OT in BET and demonstrated that the operation time in VATS was shorter than thoracotomy (97 vs. 155 min; P<0.001) with fewer postoperative complications, such as atelectasis, prolong air-leak, ventilator dependence and reintubation (10). Cardillo et al. performed a retrospective cohort study and demonstrated that VATS had a better results compare to OT in terms of in-hospital postoperative pain (day 1 and 7) (5 vs. 6; P<0.0001), postoperative air leak (2.8±2.4 vs. 3.9±4.3 days; P=0.004), operative time (70±7.4 vs. 76.9±6.8 min; P<0.0001), hospital stay (8.6±1.8 vs. 10±7.8 days; P=0.020) and time to return to work (25±5.2 vs. 34.1±9.9 days; P<0.0001) (29). Another study was described by Casali, they showed a benefit of VATS superior than OT in lower postoperative hospital stay (6.4 vs. 9 days; P=0.008) and shorter duration of chest drainage (4 vs. 7.3 days; P=0.006) (30). Our study was also shown a benefit in less post-operative complication outcome especially in re-intubation, wound infection, duration of ICU stays and ventilator dependence.
Based on our result, VATS is a safe and effective approach for empyema thoracic with success rate of 92.9% in complete decortication and 89.9% in full lung expansion after operation. Median operative time was 105 min. An incidence of composite complication and mortality rate were 19.39% and 9.2%, respectively. This result is comparable with previous studies (7,18,26,31). However, there are some limitations of this study. It was retrospective nature, the sample size of this study is not enough to perform a propensity match analysis, this is a reason why we perform multilevel stratified by PS. Some important variables such as number of loculation, thickness of visceral pleural peel, body mass index or weight, or presence of calcified peel which may affect the selection of approach are not available for analysis. A prospective randomized controlled trial with larger sample size is warranted to confirm the equivalent results between the two surgical approaches.
Conclusions
VATS decortication is a feasible surgical treatment for bacterial empyema thoracic which could decrease incidence of re-intubation rate, wound infection rate, duration of ICU stays and ventilator dependence. However, whenever adequate decortication cannot achieve in VATS approach, conversion to thoracotomy should be considered.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kamran Ali) for the series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.12.14). The series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by local research ethics committee in each hospital (No. 136/61). Individual informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alfageme I, Munoz F, Pena N, et al. Empyema of the thorax in adults. Etiology, microbiologic findings, and management. Chest 1993;103:839-43. [Crossref] [PubMed]
- Brook I, Frazier EH. Aerobic and anaerobic microbiology of empyema. A retrospective review in two military hospitals. Chest 1993;103:1502-7. [Crossref] [PubMed]
- Ferguson AD, Prescott RJ, Selkon JB, et al. The clinical course and management of thoracic empyema. QJM 1996;89:285-9. [Crossref] [PubMed]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865-74. [Crossref] [PubMed]
- Smith JA, Mullerworth MH, Westlake GW, et al. Empyema thoracis: 14-year experience in a teaching center. Ann Thorac Surg 1991;51:39-42. [Crossref] [PubMed]
- Weissberg D, Refaely Y. Pleural empyema: 24-year experience. Ann Thorac Surg 1996;62:1026-9. [Crossref] [PubMed]
- Kim BY, Oh BS, Jang WC, et al. Video-assisted thoracoscopic decortication for management of postpneumonic pleural empyema. Am J Surg 2004;188:321-4. [Crossref] [PubMed]
- St Peter SD, Tsao K, Spilde TL, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg 2009;44:106-11; discussion 11. [Crossref] [PubMed]
- Striffeler H, Gugger M. Video-assisted thoracoscopic surgery for fibrinopurulent pleural empyema in 67 patients. Ann Thorac Surg 1998;65:319-23. [Crossref] [PubMed]
- Tong BC, Hanna J, Toloza EM, et al. Outcomes of video-assisted thoracoscopic decortication. Ann Thorac Surg 2010;89:220-5. [Crossref] [PubMed]
- Yu H. Management of pleural effusion, empyema, and lung abscess. Semin Intervent Radiol 2011;28:75-86. [Crossref] [PubMed]
- Striffeler H, Ris HB, Wursten HU, et al. Video-assisted thoracoscopic treatment of pleural empyema. A new therapeutic approach. Eur J Cardiothorac Surg 1994;8:585-8. [Crossref] [PubMed]
- Hamm H, Light RW. Parapneumonic effusion and empyema. Eur Respir J 1997;10:1150-6. [Crossref] [PubMed]
- Reichert M, Hecker M, Witte B, et al. Stage-directed therapy of pleural empyema. Langenbecks Arch Surg 2017;402:15-26. [Crossref] [PubMed]
- Davies CW, Gleeson FV, Davies RJ, et al. BTS guidelines for the management of pleural infection. Thorax 2003;58:ii18-28. [Crossref] [PubMed]
- Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32. [Crossref] [PubMed]
- Howington JA, Gunnarsson CL, Maddaus MA, et al. In-hospital clinical and economic consequences of pulmonary wedge resections for cancer using video-assisted thoracoscopic techniques vs traditional open resections: a retrospective database analysis. Chest 2012;141:429-35. [Crossref] [PubMed]
- Shahin Y, Duffy J, Beggs D, et al. Surgical management of primary empyema of the pleural cavity: outcome of 81 patients. Interact Cardiovasc Thorac Surg 2010;10:565-7. [Crossref] [PubMed]
- Laohathai S, Attanawanich S, Ngodngamtaweesuk M, et al. VAT decortication. Asvide 2019;6:006. Available online: http://www.asvide.com/article/view/29470
- Sahn SA. An undiagnosed pleural effusion. Hosp Pract (Off Ed) 1993;28:60-4, 7; discussion 7-8.
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006;3:75-80. [Crossref] [PubMed]
- Bender JM, Ampofo K, Sheng X, et al. Parapneumonic empyema deaths during past century, Utah. Emerg Infect Dis 2009;15:44-8. [Crossref] [PubMed]
- Chen W, Lin YC, Liang SJ, et al. Hospital-acquired thoracic empyema in adults: a 5-year study. South Med J 2009;102:909-14. [Crossref] [PubMed]
- Rogers ML, Duffy JP. Surgical aspects of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 2000;18:711-6. [Crossref] [PubMed]
- Scarci M, Abah U, Solli P, et al. EACTS expert consensus statement for surgical management of pleural empyema. Eur J Cardiothorac Surg 2015;48:642-53. [Crossref] [PubMed]
- Angelillo Mackinlay TA, Lyons GA, Chimondeguy DJ, et al. VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema. Ann Thorac Surg 1996;61:1626-30. [Crossref] [PubMed]
- Wait MA, Sharma S, Hohn J, et al. A randomized trial of empyema therapy. Chest 1997;111:1548-51. [Crossref] [PubMed]
- Farjah F, Backhus LM, Varghese TK, et al. Ninety-day costs of video-assisted thoracic surgery versus open lobectomy for lung cancer. Ann Thorac Surg 2014;98:191-6. [Crossref] [PubMed]
- Cardillo G, Carleo F, Carbone L, et al. Chronic postpneumonic pleural empyema: comparative merits of thoracoscopic versus open decortication. Eur J Cardiothorac Surg 2009;36:914-8. [Crossref] [PubMed]
- Casali C, Storelli ES, Di Prima E, et al. Long-term functional results after surgical treatment of parapneumonic thoracic empyema. Interact Cardiovasc Thorac Surg 2009;9:74-8. [Crossref] [PubMed]
- Lawrence DR, Ohri SK, Moxon RE, et al. Thoracoscopic debridement of empyema thoracis. Ann Thorac Surg 1997;64:1448-50. [Crossref] [PubMed]
Cite this article as: Laohathai S, Attanawanich S, Ngodngamtaweesuk M, Samankatiwat P, Cherntanomwong P, Aeesoa S, Suvarnakich K, Sadade S, Innipat T, Tangpiroontham P, Homvises B, Thongjareon P, Saeteng S, Siwachat S, Taioli E, Tantraworasin A. Video-assisted thoracoscopic surgery in bacterial empyema thoracic result from developing country based on Thailand experience. J Vis Surg 2019;5:7.