Role of surgery in the management of necrotizing pneumonia
Introduction
Necrotizing pneumonia (NP) is an uncommon and severe complication of community-acquired pneumonia (CAP) which is generally associated with poor clinical outcomes. Complicated CAP can present as lung abscess, NP and lung gangrene based on the extent of parenchymal involvement, degree of necrosis and inflammation, severity of sepsis and radiological features. NP usually manifests with sepsis and rapidly progressive acute respiratory failure. Radiologically, it is characterized by pulmonary inflammation with consolidation, necrosis and multiple small cavities (1). This is often accompanied by some degree of vascular compromise contributing to failure of medical therapy resulting from inadequate antibiotic delivery (2,3).
No clear guidelines exist for management of such patients. However surgery is sometimes offered upon failure of adequate medical management, often as a life saving measure. The indications are persistent or major hemoptysis, empyema, abscess and lung gangrene (1).
Case presentation
We searched the electronic database of our department between October 2016 and October 2018, using the key word “necrotizing pneumonia” in the diagnosis section. A retrospective review of the case records of all patients of NP who underwent a resectional lung surgery was then performed (Tables 1,2).
Table 1
| Case | Age (years) | Sex | Sepsis | Empyema | Air-leak | Ventilatory support | Pathogen isolated from pleural/lung |
|---|---|---|---|---|---|---|---|
| 1 | 24 | Male | + | + | + | + | Acinetobacter baumanii |
| 2 | 5 | Female | + | + | − | − | Streptococcus pneumoniae |
| 3 | 27 | Female | + | + | + | + | Mycobacterium tuberculosis, Staphylococcus aureus |
| 4 | 37 | Female | + | + | + | − | Methicillin resistant Staphylococcus aureus, Burkholderia cepacia |
| 5 | 41 | Male | + | + | + | − | Staphylococcus aureus |
| 6 | 22 | Male | + | − | − | + | Streptococcus pneumoniae |
Table 2
| Case | Time from admission to surgery (days) | Approach | Laterality | Procedure | Reoperations | Death |
|---|---|---|---|---|---|---|
| 1 | 19 | Open | Right | Bilobectomy (right upper and middle), wedge resection (right lower), decortication | − | + |
| 2 | 13 | Open | Left | Wedge resection (left upper) | − | − |
| 3 | 8 | Open | Left | Left upper lobectomy, decortication | − | − |
| 4 | 22 | Open | Right | Bilobectomy (right upper and middle), decortication | − | − |
| 5 | 7 | Open | Right | Right upper lobectomy | − | − |
| 6 | 11 | VATS | Right | Right lower lobectomy | − | − |
VATS, video-assisted thoracoscopic surgery.
NP was defined as patchy inflammation with microabscesses and a lack of perfusion on computed tomography (CT) (4,5). Scans of all our patients were reviewed and checked for multiple areas of lung parenchyma containing air, air and fluid or non-enhancing fluid surrounded by contrast enhancing lung parenchyma without a defined rim of enhancement.
Sepsis was defined as systemic response to infection and was defined as the presence of systemic inflammatory response syndrome (SIRS) in addition to a documented or presumed infection. Criteria for SIRS: Heart rate >90 beats/minute, Respiratory rate >20 breaths/minute, temperature >38 or <36 degree Celsius, White blood cells >12,000/mm3, <4,000/mm3, or >10% bands.
Case 1
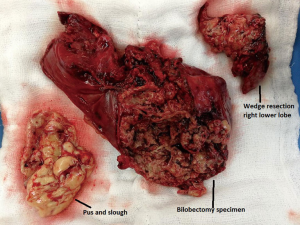
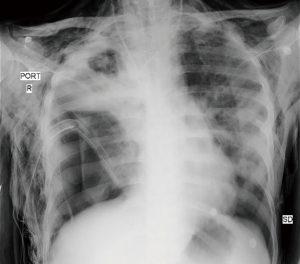
A 24-year-old male, status post liver transplant for Wilson’s disease (with steroid resistant acute rejection), was presented to a specialized liver disease hospital with sudden onset shortness of breath and fever. On chest X-ray he was found to have right hydropneumothorax. Intercostal chest drain was placed on right side. Sputum was positive for acid fast bacilli (AFB) and he was started on ATT. Sputum culture also grew Acinetobacter baumanii. He was managed conservatively for about 15 days but on failure of improvement was referred to our centre for further management. Chest X ray (Figure 1) and CT thorax on initial evaluation at our centre revealed features of extensive NP of right lung and empyema and a right sided pneumothorax. Thoracotomy was done and grossly necrotic and gangrenous right upper lobe and middle lobe resected. Wedge resection of apical segment of lower lobe was also done along with decortication (Figure 2). Biopsy confirmed NP. In the post-operative period patient developed coagulopathy and needed multiple blood and blood product transfusions including fresh frozen plasma, cryoprecipitate and platelets. He was weaned off ventilator on post op day 2 and inotropic support gradually withdrawn. Empyema fluid grew multidrug resistant Acinetobacter. His pancytopenia persisted and a bone marrow biopsy revealed hemophagocytic lymphohistiocytosis. On post op day 9 he developed massive air leak from right chest drain and complete wound dehiscence, desaturated and required inotropic support again. On day 12 of surgery he succumbed to septic shock and multi organ failure.
Case 2
A 5-year-old girl presented to the emergency department of her local hospital with a 10-day history of weakness and cough. She was diagnosed with pneumonia and discharged home with antibiotics. However three days later, she presented again to the hospital in respiratory distress. On examination, she was febrile, hypotensive, had flaring of alae nasi and intercostal recession. She was intubated, placed on vasopressors and empirical antibiotics. Blood cultures taken in emergency, grew penicillin-sensitive Streptococcus pneumoniae. CT thorax demonstrated left-sided empyema, small pneumothorax, necrosing pulmonary tissue and consolidation. Within the consolidated LUL were multiple areas resembling microabscesses. A chest tube was inserted for the left-sided pyo-pneumothorax by the paediatrician and thoracic surgery reference was sought. She underwent a wide wedge resection of the left upper lobe and drainage of empyema, the next day. She recovered well and was discharged on post-op day five.
Case 3
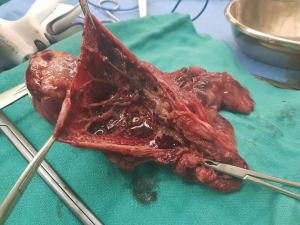
A 27-year-old female with a history of fever on and off for last 3 months (for which she was taking alternative medicine Ayurvedic treatment without much relief), developed increasingly worsening shortness of breath for 7 days and was admitted to a speciality hospital in the city. CT scan revealed left hydro-pneumothorax and consolidated left lung upper lobe. A chest drain was placed and she was started on anti-tubercular treatment (ATT). However the chest drain showed massive air leak and drained copious amounts of purulent pus. Her condition deteriorated, her blood pressure dropped, fever persisted and she developed altered sensorium. She was referred to our centre in this state and was found to have sepsis, septic shock, meningitis along with severe malnutrition. After stabilization, she underwent a left posterolateral thoracotomy. On exploration she was found to have a necrosed and ruptured cavity in the apex of the left lung upper lobe, densely adherent to chest wall. Large amount of air was found leaking from a bronchopleural fistula formed through the ruptured lung (Figure 3). Rest of the upper lobe was densely consolidated. Left upper lobectomy was performed. Chest drain was removed on post-op day 7 because of prolonged air leak and she was discharged on post-op day 12.

Case 4
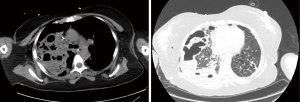
A 37-year-old post-partum female, presented to the pulmonology department of our hospital with cough, hemoptysis, shortness of breath and fever for last 3 days. On admission she had severe tachycardia, tachypnoea, low oxygen saturations on ambient air (71%) and bilateral crepitations on auscultation. Next day she developed type 2 respiratory failure and right sided pneumothorax. She was intubated and right chest drain was placed. CT scan chest showed extensive consolidation in right lung upper lobe and middle lobe with multiple cavitary changes and evidence of lung destruction (Figure 4A,B). Echocardiography revealed mobile vegetation on anterior leaflet of tricuspid valve. Endotracheal secretion culture grew Methicillin resistant Staphylococcus Aureus (MRSA) and pleural fluid grew Burkholderia. Antibiotic optimization was done as per antibiogram. 2nd chest drain was placed in view of persistent air leak and increasing pneumothorax on chest radiograph. However that did not help and she continued to deteriorate. She was referred to our team for a possible surgical intervention.
On 15th day of admission she was taken for surgery. There was gross right empyema thoracis. Right upper lobe and middle lobe were found to be necrosed and discharging pus. Lower lobe was entrapped in a thick cortex. Empyema was drained, Bilobectomy (Right upper and middle) was done along with decortication of right lower lobe and tracheostomy. There was initial improvement after the surgery, lung expanded well and air leak stopped. However the pleural fluid isolate this time grew multi drug resistant Burkholderia. Patient succumbed to sepsis on day six of surgery.
Case 5
A 41-year-old diabetic male was referred from a tier 2 city in North India to our centre with community acquired pneumonia not responding to conservative treatment of about 7 days. Upon evaluation his bronchoalveolar lavage was positive for pan-sensitive Staphylococcus. CT of the thorax revealed extensive consolidation of the right lung, with multiple cavities (largest 5.0 cm × 7.0 cm) in right upper lobe, along with extensive necrosis. Piperacillin-Tazobactum was started and he was referred to us for surgical management. He underwent a Video-assisted thoracoscopic resection of the right upper lobe (Figure 5). He responded well to the surgery. The drain came out on post op day 4 and had good radiological and clinical resolution.
Case 6
A 22-year-old young male presented to the emergency section of our hospital with fever, shortness of breath and streaky hemoptysis of 3 day duration. He was diagnosed with pneumonia and was admitted. Bronchoscopy was done and lavage taken. He was started on empirical antibiotics to which he did not respond well. On 6th day of admission he became severely short of breath and acidotic. He ultimately got intubated. CT thorax revealed extensive right lower lobe pneumonia with cavitation and necrosis. Cultures revealed Streptococcus pneumoniae and antibiotics were escalated as per the antibiogram. However he did not show significant clinical improvement and Thoracic surgery consultation was sought. He was taken up for a right lower lobectomy via a posterolateral thoracotomy on day 11 of admission. He tolerated the surgery well and was extubated on post-operative day 1. He was discharged on Post-operative day 6 after drain removal.
Discussion
In all three categories of necrotizing lung infection the onset of disease is marked by the classical symptomatology of pneumonia. Later on the clinical course and radiological appearance may change dramatically based on a number of factors like general health and immunity of the patient, type of micro-organism and it’s virulence, delays in getting medical therapy, inappropriate antibiotics or antibiotic resistance, inadequate pleural drainage procedures etc.
The predominant organisms associated with complicated NP or lung gangrene are Klebsiella pneumoniae, P aeruginosa, S aureus and S pneumonia (1,7). In paediatric population, the most common pathogen is S pneumoniae.
Preoperative imaging studies are crucial in management of NP. It is justified to be liberal in repeating the CT scans to check for progression of disease, monitor the response to medical therapy, to guide drainage procedures and to make a call regarding a possible surgical intervention. Usual findings on a CT scan of chest would reveal some degree of parenchymal destruction, associated pleural empyema, cavitary changes, occult findings like microabscesses, and areas of hypoperfusion or no perfusion (4,8). As the infection progresses, necrotic areas coalesce to form a single or few large cavities containing sloughed lung tissue that floats atop pus collected in the bottom of the abscess. In the final stage a large mass of necrotic lung tissue can cause respiratory distress and pneumothorax (9). Reimel et al. in their series of 17 patients of NP observed that in cases where CT with contrast showed that majority of lung parenchyma was perfused, had resolving sepsis, did not require surgery and could be followed up with interval CT scans to look for resolution (10).
It is difficult to decide which patients of NP would need surgery and which one’s could be managed by medical management. However accepted indications for surgical resection of pulmonary parenchyma in necrotizing lung infections include massive hemoptysis, lung gangrene and NP not responding to medical therapy with progressive parenchymal destruction (1,2,11,12). Schamaun et al. reported on 14 patients with massive unilateral gangrene. Four of these patients were treated medically alone and all four died. The other 10 patients underwent surgical resection and all survived (13).
But how long should one wait in NP not responding to medical management? The ideal timing for surgery is unclear. Reimel and colleagues are in favour of waiting until patients have been medically stabilized which allows for better surgical outcomes (10). According to the authors delaying surgery provides time for “areas of lung that had documented perfusion to resolve, further localizing the area that actually required resection”. Chatha et al. (14) in their case series of NP however feel that in delaying the surgical intervention, the patient’s condition may worsen in the interim. Two patients in their series who died had a significant delay in thoracic surgery consultation (11 days and 18 days after initial presentation). The authors raise an important question of whether there may have been an earlier time window during which surgical intervention may have resulted in better outcomes. They also pointed out that bacterial contamination of contralateral lung may convert a unilateral pneumonia into a bilateral disease. In our series we found a similar pattern to Chatha et al. wherein two mortalities were observed out of six patients when the time between admission and surgery was more than 2 weeks. Few authors consider treating empyema before lung resection to stabilize patients with acute sepsis requiring resuscitation (14,15) including staged “fenestration” procedures (16).
When one goes down the operative route, the goals should be to control the ongoing sepsis, drain the empyema, unroof lung abscesses, resect or debride necrotic tissue, re-expand the lung and protect the contralateral lung from spillage while trying to avoid large spatial voids and large bronchopleural fistulas (10). There is no ideal surgical procedure for all patients of NP and the procedure has to be customized in each individual. The surgical procedures can range from necrosectomy to decortications to pulmonary resections (wedge resection, lobectomy, pneumonectomy) depending on the given clinical situation, assessment of CT scans and intraoperative findings. Lai et al. (9) operated on 56 patients of necrotizing pneumonitis with empyema in children. They divided the study group into uncomplicated and complicated NP. CT finding of massive lung necrosis or large cavities >50% of the involved lobe were deemed to be complicated NP. They found in their study that aggressive surgical treatment results in significant clinical improvement and lobectomy in patients with complicated NP may shorten the postoperative course and avoid subsequent surgery.
Most surgeons resort to a traditional thoracotomy approach for NP with no hesitation to divide more than one rib to gain the desired exposure (10). Video-assisted thoracoscopic surgery (VATS) is not recommended by majority of them as these patients often do not tolerate single lung ventilation (1,10,14). However one patient in our series was managed by a VATS lobectomy. Intraoperatively according to Reimel et al. the key technical issues are to identify the pulmonary artery early, which, in the case of lung gangrene, is surprisingly easy because the parenchymal tissue tends to shred away and the pulmonary artery in the fissure can be found with simple blunt dissection. NP without frank gangrene is more difficult because the parenchyma is heavy and dense, and in many cases, it is easier to ligate the pulmonary vein first; this allows the lung to be retracted with greater ease to identify either the proximal main pulmonary artery or the artery in the fissure proximal to the lower lobe on either side, which then allows dissection into the fissure (10). The authors also advocate bronchial stump reinforcement with viable tissue especially after a pneumonectomy.
Chatha et al. (14) add a word of caution for the physicians and surgeons that these patients may worsen after surgery before they get better. The systemic inflammatory response may also be augmented by surgery, therefore clinicians must be prepared for the possibility of unstable hemodynamics intra- and post-operatively. They also propose protective lung ventilation strategies as the lung would be contused post-operatively with further inflammation and capillary leak. High mortality rates have been reported in several retrospective studies ranging from 9% to 20% (10,12). Schweigert and colleagues (17) reported a mortality rate of 15.9% in patients of NP with gangrene. In their series, two patients out of eleven died after a pneumonectomy and five out of twenty six died after undergoing a lobectomy whereas all patients following sublobar resections survived. Two out of six patients undergoing a lung resection died in our study group as well.
Patients with preoperative endotracheal intubation, hemolytic uremic syndrome, preoperative pneumothorax and diffuse bilateral parenchymal involvement have the worst prognosis (9,10).
Conclusions
NP carries significant morbidity and mortality. In our experience and in the light of available literature early thoracic surgery consultation should be sought to assess patients not responding well to appropriate medical management which may potentially benefit from a surgical intervention. Surgery for NP has to be tailor-made for different set of patients with varying degree of pleural and parenchymal involvement.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.12.15). The series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases” was commissioned by the editorial office without any funding or sponsorship. KA served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). The study is approved by our institutional ethical committee and detailed informed consent was obtained from every patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Krishnadasan B, Sherbin VL, Vallieres E, et al. Surgical management of lung gangrene. Can Respir J 2000;7:401-4. [Crossref] [PubMed]
- Curry CA, Fishman EK, Buckley JA. Pulmonary gangrene: Radiological and pathologic correlation. South Med J 1998;91:957-60. [Crossref] [PubMed]
- Gutman E, Rao KV, Park YS. Pulmonary gangrene with vascular occlusion. South Med J 1978;71:772-5. [Crossref] [PubMed]
- Donnelly LF, Klosterman LA. Pneumonia in children: decreased parenchymal contrast enhancement-CT sign of intense illness and impeding cavitary necrosis. Radiology 1997;205:817-20. [Crossref] [PubMed]
- Penner C, Maycher B, Long R. Pulmonary gangrene. A complication of bacterial pneumonia. Chest 1994;105:563-73. [PubMed]
- Ali K, Bal S, Mobashir A. Intraoperative video showing significant air-leak from devitalized left lung upper lobe. Asvide 2019;6:007. Available online: http://www.asvide.com/article/view/29495
- Moon WK, Im JG, Yeon KM, et al. Complications of Klebsiella pneumonia: CT evaluation. J Pediatr Surg 1995;19:176-81. [Crossref] [PubMed]
- Cowles RA, Lelli JL Jr, Takayasu J, et al. Lung resection in infants and children with pulmonary infections refractory to medical therapy. J Padiatr Surg 2002;37:643-7. [Crossref] [PubMed]
- Lai JY, Yang W, Ming YC. Surgical management of complicated necrotizing pneumonia in children. Pediatr Neonatol 2017;58:321-7. [Crossref] [PubMed]
- Reimel BA, Krishnadasen B, Cuschieri J, et al. Surgical management of acute necrotizing lung infections. Can Respir J 2006;13:369-73. [Crossref] [PubMed]
- Knight L, Fraser RG, Robson HG. Massive pulmonary gangrene: A severe complication of Klebsiella pneumonia. Can Med Assoc J 1975;112:196-8. [PubMed]
- Karmy-Jones R, Vallieres E, Harrington R. Surgical management of necrotizing pneumonia. Clin Pulm Med 2003;10:17-25. [Crossref]
- Schamaun M, von Buren U, Pirozynski W. Massive lung necrosis in klebsiella pneumonia (so-called massive lung gangrene). Schweiz Med Wochenschr 1980;110:223-5. [PubMed]
- Chatha N, Fortin D, Bosma KJ. Management of necrotizing pneumonia and pulmonary gangrene: A case series and review of the literature. Can Respir J 2014;21:239-45. [Crossref] [PubMed]
- Odell JA, Buckels NJ. Techniques of pneumonectomy. Pneumonectomy through an empyema. Chest Surg Clin N Am 1999;9:369-78. x-xi. [PubMed]
- Refaely Y, Weissberg D. Gangrene of the lung: Treatment in two stages. Ann Thorac Surg 1997;64:970-4. [Crossref] [PubMed]
- Schweigert M, Dubecz A, Beron M, et al. Surgical therapy for necrotizing pneumonia and lung gangrene. Thorac Cardiovasc Surg 2013;61:636-41. [PubMed]
Cite this article as: Ali K, Bal S, Mobashir A. Role of surgery in the management of necrotizing pneumonia. J Vis Surg 2019;5:8.