Management of hemorrhage during thoracoscopic surgery for anterior mediastinal tumors
Introduction
Several important organs, including the aorta, superior vena cava, right and left brachiocephalic veins, heart, vagus, and phrenic nerves, are located in the mediastinum or in its immediate vicinity. When performing mediastinal tumor surgeries, great care is needed so as not to damage these structures. In particular, any damage to the left brachiocephalic vein during thymectomy for anterior mediastinal tumors or myasthenia gravis may result in serious consequences. Recently, thoracoscopic surgery for mediastinal tumors has become a frequent procedure but is generally associated with more difficult management of hemorrhage than open surgery; therefore, extra care is needed for ensuring safety. Here we discuss the selection of the endoscopic surgery approach when performing surgeries for anterior mediastinal tumors from the perspective of patient safety and describe preparations for and management of vascular damage during endoscopic surgeries, with particular emphasis on damage to the left brachiocephalic vein.
Selection of endoscopic surgery approach for anterior mediastinal tumors
Recently, endoscopic surgery has been performed for anterior mediastinal tumors using cervical, lateral thoracic, and subxiphoid approaches (1-4). Although the lateral thoracic approach has come into widespread use worldwide, in recent years, the subxiphoid approach has also been studied (4,5). Regardless of the surgical approach, special considerations are needed during endoscopic surgery to ensure patients’ safety. In particular, indications for endoscopic surgery based on tumor size and whether the tumor has invaded the surrounding organs need to be adhered to. In the lateral approach, extraction of large solid tumors through the narrow intercostal space is difficult. There are no ribs in the pass from beneath the xiphoid process to the anterior mediastinal area; therefore, even relatively large tumors can be removed by extending the wound. The author has experienced extraction of a tumor of 12 cm diameter through the wound below the xiphoid process. The maximum sized tumor that can be extracted through a wound under the xiphoid process is unknown; however, based on the author’s experience, extraction of a tumor of approximately 20-cm diameter may be possible.
In cases in which the tumor is large and makes it difficult to visualize the exact location of the left brachiocephalic vein and those in which the tumor may have invaded the left brachiocephalic vein, defensive preparations should be made against any damage to the left brachiocephalic vein during surgery. This is done by performing endoscopic clamping of the brachiocephalic vein using clamp forceps and taping the central portion of the tumor and the peripheral end of the brachiocephalic vein as quickly as possible. However, when using the lateral thoracic approach, it is impossible to view the distal side of a tumor in the left brachiocephalic vein if the tumor has invaded the left brachiocephalic vein, and the surgery is deemed too risky. In contrast, when using the subxiphoid approach, the camera is inserted from the midline of the body and allows for the visualization of the left brachiocephalic vein from its central portion to its periphery, which allows for taping (although taping is also dependent upon tumor location). If the tumor is in contact with the left brachiocephalic vein or the endoscopic surgery is to be performed on a case with a suspected tumor invasion, then the subxiphoid approach is a valid option. Another approach would be one using robotic surgery, considering the need for both taping and suturing (6). Robotic surgery devices with joints that move in the same way as a human hand, which can be operated in narrow spaces, allow for more advanced surgical procedures than would be possible for human surgeons alone. In my personal experience, robot-assisted thymectomy with the subxiphoid approach should be indicated for cases in which the tumor is in contact with the brachiocephalic vein and those in which the tumor is located on the cranial side of the brachiocephalic vein because it allows for easier taping of the brachiocephalic vein and easier suturing of the fine blood vessels. Therefore, this approach has the widest range of applications during endoscopic surgery.
However, when perivascular dissection is difficult and unsafe, this procedure should not be performed. The criterion for determining whether endoscopic surgery is indicated for an anterior mediastinal tumor is based on whether the tumor can be safely dissected from the left brachiocephalic vein. The range of applications differs depending upon the surgical approach selected.
Preoperative preparations
Surgery for anterior mediastinal tumors is performed in the vicinity of the heart, aorta, brachiocephalic artery, superior vena cava, and left and right brachiocephalic veins. Thus, damage to any of these structures leads to extensive hemorrhage and a crisis situation. To prepare for the possibility of vascular damage, the surgical team, including the surgeon, anesthesiologist, and surgical nurses, should be assembled to check the instruments required and procedures to be used if hemorrhage occurs. Our preoperative preparations for hemorrhage include the following: (I) ensuring that TachoSil® (Takeda Austria GmbH, Linz, Austria), a fibrin sheet useful during transfusions and compression hemostasis, is present in the operating room (OR) and that it can be immediately used; (II) a sternum saw, rib spreaders, and vascular clamp forceps are present in the OR; (III) procedures in case of hemorrhage are confirmed by the entire team (surgeon, anesthesiologist, and surgical nurses) prior to the start of the surgery; and (IV) being tape the peripheral and central sides of a site of invasion. In cases in which the tumor is found to be firmly adhered to the blood vessel during surgery, it can be continued only after taping the peripheral and central sides of the site of invasion and those in which surgical procedures become difficult to perform as a result, conversion to open surgery (median sternotomy) should be done (Table 1).
Table 1
| Blood for transfusion and TachoSil® should be ready for use whenever necessary |
| The surgical team (surgeon, anesthesiologist, and nurses) should discuss the procedure for hemorrhage management prior to the start of the procedure |
| A sternum saw, rib spreaders, and vascular clamp forceps should be made readily available in the OR to facilitate emergency median sternotomy in case of hemorrhage |
| In cases with possible tumor invasions in the left brachiocephalic vein or the superior vena cava, the areas central and peripheral to the invasion site should be taped prior to continuation with the surgical procedure. In cases in which this is difficult, conversion to open surgery should be done |
OR, operating room.
Anterior mediastinal tumor surgery is performed with the patient in the lateral recumbent position in some facilities and in the decubitus or 30-degree semi-lateral decubitus position in others. In cases with damage to and hemorrhage from the left brachiocephalic vein or any of the thymic veins (tributaries of the left brachiocephalic vein), compression hemostasis is first attempted, but if hemostasis is not achieved, then thoracotomy and clamping of the central and peripheral portions of the damaged site of the left brachiocephalic vein become necessary. In the lateral decubitus position, when hemostasis is attempted during thoracotomy, intercostal thoracotomy of the lateral chest should be performed to compress the damaged area. However, it is impossible to clamp the distal part of the left brachiocephalic vein injury during compression from the lateral chest. If clamping of the left brachiocephalic vein is essential, the patient’s posture must be changed from the lateral decubitus to supine position. However, changing the patient’s posture while compressing deep areas in the thoracic cavity by hand can cause displacement of the hand or the tool used for compression, which may lead to re-bleeding. It may also lead to displacement of the double-lumen endotracheal tube, causing failure to maintain unilateral ventilation. These conditions may make it difficult to continue to accurately compress the injured vein. Therefore, thymectomy in the lateral position is dangerous, considering the response to left brachiocephalic vein injury. When performing surgery for an anterior mediastinal tumor, which requires that the procedure be performed in the vicinity of the left brachiocephalic vein, or in cases of myasthenia gravis, the patient should be in the supine or semi-supine position because this allows for swift conversion to median sternotomy. Also, the intravenous route in a peripheral vessel should be confirmed prior to surgery, and considering that it may be necessary to clamp the left brachiocephalic vein if it suffers damage, the intravenous route should not be in the left upper arm but rather in the right upper arm or leg.
Hemorrhage management
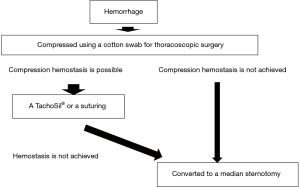
Hemorrhage management is more difficult during endoscopic surgery than during open surgery. Thus, safety considerations must precede the procedure. The following is our procedure for hemorrhage management (Figure 1):
- Compression of the hemorrhage site;
It is important not to panic and become disorganized when hemorrhage occurs. Apply compression using a cotton swab or gauze designed for use in thoracoscopic surgery. If bleeding is managed by compression, and the surgical team comprises insufficient numbers of surgeons, anesthesiologists and nurses, compression should be maintained until a surgical team can be assembled, and sufficient blood can be prepared for transfusion. The volume of blood to be prepared for transfusion depends on the situation. If bleeding is severe, to the extent that vertical incision of the sternum is necessary, the volume of blood required becomes larger. Once the additional team members are in place and preparations for transfusion and TachoSil® are completed, move on to the hemostasis procedure. If hemostasis is not achieved through compression with cotton swabs or gauze, then swiftly convert the procedure to a median sternotomy.
- Thoracoscopic hemostasis methods;
During single-port-thymectomy via subxiphoid approach, if suturing is necessary, a 12 mm port with a valve should be inserted at the right fifth intercostal space on the anterior axillary line to prevent leaking of CO2 distribution pressure. This can be inserted from the left side; however, care should be taken to not damage the heart, which is close to the chest wall in this approach.
A TachoSil® Fibrin Sealant Patch can be put in place with generally good results. Once compression of the hemorrhage site is achieved using cotton swabs and gauze for use in thoracoscopic surgery, prepare the TachoSil®. I cut a single sheet into eight equally sized pieces prior to use. If the damage is minute and there is only a small amount of bleeding, then the sheet can be cut into 32 equally sized pieces. In cases in which the surgeon is compressing the hemorrhage site, the surgical assistant uses tweezers to grasp the TachoSil® and carry it to the vicinity of the hemorrhage site. If a port with a valve for the purpose of CO2 feed is in use, then wrapping the TachoSil® in a small piece of gauze prior to insertion will allow it to pass the valve without causing any damage. The surgeon then releases the compression, and the assistant swiftly places the TachoSil® onto the hemorrhage site. The surgeon then uses the above-mentioned type of cotton swab or gauze to press the TachoSil® onto the hemorrhage site and apply pressure. If hemostasis is incomplete, then the same procedure is repeated. Hemostasis using sutures is another option. Perform hemostasis using endoscopic Z-suturing of the hemorrhage site (Figure 2).

- Hemostasis via median sternotomy;
If thoracoscopic hemostasis is deemed difficult, then swiftly convert to median sternotomy without any hesitation or delay, and safely performing the procedure is of utmost importance. Clamp the peripheral and central regions of the damaged site of the left brachiocephalic vein and perform hemostasis with sutures. If the damaged vessel is the superior vena cava, then extracorporeal circulation is useful for reducing the removal of right heart blood to the pump and amount of bleeding.
Comments
The unintentional damage to the left brachiocephalic vein during surgery for anterior mediastinal tumor is a life-threatening complication. In most cases in which the hemorrhage originates from the left brachiocephalic vein, hemostasis can be effectively achieved using compression and TachoSil®. However, if these methods do not achieve hemostasis, then the left brachiocephalic vein must be clamped. Because it is difficult to clamp this vessel when the patient is in the lateral decubitus position, surgery should be performed with the patient in the supine position, which allows for conversion to median sternotomy regardless of the endoscopic surgery approach being used. In cases in which the anterior mediastinal tumor is suspected to have invaded and in contact with the left brachiocephalic vein, dissection of the tumor from the brachiocephalic vein is required. However, to ensure that this is safely performed, preparations should be made for potential hemorrhaging, and it is advisable to perform taping of the brachiocephalic vein at the central and peripheral portions of the tumor. However, when using the most common lateral thoracic approach, the tumor blocks the view of the distal portion of the left brachiocephalic vein, making the taping impossible. Thus, for these cases, endoscopic surgery is not recommended because of safety concerns. Lowering the distribution pressure of CO2 has been reported, in animal experiments, as an alternative to suturing or the use of fibrin sheets for hemostatic management of venous bleeding (8). However, application of this method to human surgery must be validated in future. We set the distribution pressure of CO2 to 8 mmHg during thymectomy via subxiphoid approach. Risk of embolism arises if distribution pressure of CO2 becomes too high. Theoretically, it should also be possible to achieve hemostasis through distal left brachiocephalic vein occlusion using a balloon or stent inserted via a catheter from the left cubital vein to the left brachiocephalic vein. However, whether these methods are feasible during an actual surgery remains unclear.
In recent years, endoscopic surgery indications have been extended to encompass even more complicated surgical procedures. Regardless of how small the surgical wound is, the surgical procedure is not “minimally invasive” from the patient’s point of view if complications are more likely due to reduced surgical operability or if it causes hemorrhaging. Any minimally invasive surgical procedure must maintain the same or better precision than that in its open surgery counterpart and must consider patient safety.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Seshiru Nakazawa and Kimihiro Shimizu) for the series “Emergency Response to Intraoperative Bleeding” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.01.05). The series “Emergency Response to Intraoperative Bleeding” was commissioned by the editorial office without any funding or sponsorship. TS serves as an unpaid editorial board member of Journal of Visualized Surgery from Feb 2018 to Jan 2020. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from the patient for publication of this report.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cooper JD, Al-Jilaihawa AN, Pearson FG, et al. An improved technique to facilitate transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1988;45:242-7. [Crossref] [PubMed]
- Landreneau RJ, Dowling RD, Castillo WM, et al. Thoracoscopic resection of an anterior mediastinal tumor. Ann Thorac Surg 1992;54:142-4. [Crossref] [PubMed]
- Sugarbaker DJ. Thoracoscopy in the management of anterior mediastinal masses. Ann Thorac Surg 1993;56:653-6. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Suda T. Subxiphoid Robotic Extended Thymectomy with a Pericardial Patch Closure. Available online: http://www.ctsnet.org/article/subxiphoid-robotic-extended-thymectomy-pericardial-patch-closure
- Suda T. Movie showing left brachiocephalic vein injury during single-port-thymectomy via subxiphoid approach. Asvide 2019;6:026. Available online: http://www.asvide.com/article/view/29795
- Okamura R, Takahashi Y, Dejima H, et al. Efficacy and hemodynamic response of pleural carbon dioxide insufflation during thoracoscopic surgery in a swine vessel injury model. Surg Today 2016;46:1464-70. [Crossref] [PubMed]
Cite this article as: Suda T. Management of hemorrhage during thoracoscopic surgery for anterior mediastinal tumors. J Vis Surg 2019;5:13.