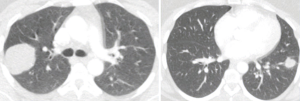
Minimally invasive approach for pulmonary hydatid cyst
Hydatid disease, that is caused by Echinococcus granulosus and Echinococcus multilocularis, is also known as echinococcosis or hydatidosis (1). The disease is endemic in a number of sheep- and cattle-raising countries, including Middle East and Mediterranean countries, New Zealand, Australia and India (1,2). Humans take part as accidental intermediate hosts and harbor cysts, which are most commonly found in the liver and lung but can be discovered in any organ (3). Pulmonary hydatid cysts rarely heal by spontaneous discharge into the bronchus. However, rupture into bronchial system, anaphylactic reaction, suffocation caused by cystic fluid are serious and possibly fatal complications (2,3). Surgical methods for treatment of pulmonary cysts include enucleation of intact cysts, and cystotomy, with or without capitonnage, for complicated or intact cysts (4). Capitonnage may shorten hospitalization time 1 day; however, the real benefit of this method remains unsolved (4). The standard management of pulmonary hydatid disease is surgical removal of cysts with or without capitonnage via thoracotomy or sternotomy in patients with bilateral disease (5,6). Preoperative evaluation of a patient with hydatid cyst of the lung should start with identifying the number and localization of the cysts. Surgical treatment for pulmonary hydatid cyst disease should include evacuation of the intact or ruptured cyst, removal of the germinative membrane, and capitonnage of the residual space (i.e., pericystic cavity) whereas preserving as much lung parenchyma as possible. The hydatid cyst disease of the lung surgery is associated with low complication and very low mortality rates (4-6).
It is fair to speculate that the greatest advance in thoracic surgery of the last 30 years has been the advent of video-assisted thoracoscopic surgery (VATS) (7). VATS has been shown to significantly reduce pain, quicken recovery, decrease complications, and improve post-operative quality of life of patients when compared to open thoracotomy (8,9). For this reason, VATS has now become a new standard of thoracic surgery. When VATS was first introduced 25 years ago, the approach typically used three ports without rib-spreading (8). There could also be an additional ‘utility’ port to extract the resected specimen. During the recent 10 years, great effort has been paid to reduce the port numbers to 3 and 2 in order to further reduce the postoperative pain and increase the quality of life (10). Performing the procedures using only one incision (uniportal VATS) was first introduced by Rocco (11). Uniportal VATS has been become eventually the major breakthrough in thoracic surgery and it was proven that most thoracic procedures can safely and perfectly be performed via uniportal VATS (12).
The minimally invasive treatment of pulmonary hydatid disease was first introduced in 1994 by Becmeur and colleagues (13). The feasibility of the procedure has been proven by others (14-23). Alpay et al. demonstrated that, VATS treatment of pulmonary hydatid disease was superior to thoracotomy causing lower pain, shorter operation time, lower chest tube drainage volume and shorter chest tube duration (19). Ma and colleagues (21) also reported that, VATS treatment of pulmonary hydatid disease in children was associated with less intraoperative blood loss, shorter chest tube duration, reduced postoperative pain and lower hospitalization cost. Ocakcioglu and Sayir (22) confirmed that, VATS is better in terms of postoperative patient-related parameters. VATS treatment follows the same principles as the thoracotomy: aspiration of the cystic fluid, instillation of scolicidal agents (diluted povidone-iodine), removal of the germinative membrane, closure of the bronchial openings, and capitonnage of the cavity. Total enucleation of unruptured cyst can be performed in open surgery, whereas, it is not safely doable during videothoracoscopic approach since small (i.e., 3–5 cm) utility incision would prevent safe extraction. It is questionable if capitonnage is necessary for pulmonary hydatid surgery. However, we previously showed that, it provides no statistically significant advantage (4), although the difference was approximately 1 day. Table 1 summarizes the studies published regarding VATS treatment of pulmonary hydatid disease.
Table 1
| Author | Year | Number of cases |
|---|---|---|
| Becmeur |
1994 | 10 |
| Paterson |
1996 | 1 |
| Mallick |
2005 | 1 |
| Parelkar |
2009 | 5 |
| Findikcioglu |
2012 | 12 |
| Alpay |
2015 | 77 |
| Ma |
2016 | 44 |
| Eroglu |
2016 | 23 |
| Bakhytzhan |
2018 | 30 |
| Ocakcioglu |
2018 | 18 |
| Total | – | 221 |
*, comparative study including open surgery.
A number of contraindications for minimally invasive treatment of pulmonary hydatid cyst were reported including giant hydatid cyst, multiple cysts and fissure or hilar location of cysts (19). However, they should not be necessarily the contraindications since multiple cysts can be dealt separately, giant cysts can be removed after aspiration of cystic fluid. Treatment of hydatid cysts at hilar location can be challenging. However, careful exploration of cyst or segmentectomy in select cases can be performed safely. Only non-feasible procedure for VATS approach in the management of pulmonary hydatid disease is enucleation of intact hydatid cyst and removal of the cyst with germinative membrane (i.e., Ugon method) since, complete extraction of unruptured cyst is physically not possible due to narrow and smaller utility incision and delicate nature of the unruptured cyst. However, there has been no publication indicating the necessity of this method as surgical management. Careful aspiration with installation of scolicidal agent around the area has been proven to be equally effective (1-4).
Uniportal approach to hydatid disease
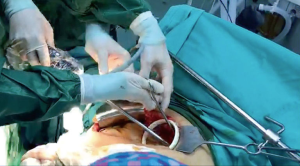
Ocakcioglu and colleagues reported that, uniportal VATS can be used for the treatment of pulmonary hydtaid disease safely (22). They reported 18 patients who had undergone this approach. We performed uniportal treatment of intact and ruptured pulmonary hydatid disease. The incision was a 3 cm long incision placed at 5th intercostal space. We used 30-degree telescope. After the lung was deflated by the usage of double-lumen intubation, the povidone-iodine soaked gauzes were placed around the cystic lesion to prevent any possible intrathoracic contamination. The cystic fluid was aspirated if the cyst was intact. using an inserted needle connected to a closed-circuit suction device (Figures 1,2). The cystic fluid was aspirated almost completely. If the cyst was ruptured seen on chest computerized tomography (CT), no aspiration was performed. The exocyst was cut using endoscopic scissors while a suction cannula was placed around and inside the cystic cavity to remove any possible spillage. The germinative membrane was removed through the incision (Figure 3). After cutting the edges of pericystic cavity, the bronchiolar openings were identified by the instillation of saline solution into the cavity while inflating the lung. The residual cavity was carefully cleaned. The bronchiolar openings were closed using 3-0 polyglactin sutures (Vicryl, Ethicon, USA). After bronchial openings were closed, the cavity was obliterated by imbricating sutures from within using 3-0 polyglactin sutures (capitonnage). With application of positive intrapulmonary pressure, any possible air leak was visualized and sealed.


After the operations, the patients were cared in service ward without a need for intensive care unit. In our practice, we performed unilateral uniportal approach for the cysts as well as consecutive operations for bilateral diseases. It was also possible to perform simultaneous bilateral subxiphoid uniportal treatment for a patient with bilateral pulmonary hydatid disease with a hepatic hydatid disease (Figures 4).
Regarding the recurrence after videothoracoscopic approach, only two recurrences were reported out of 221 patients reported so far in the literature. Alpay and associates (19) reported no recurrence in any of groups.
In conclusion, minimally invasive removal of hydatid cysts is feasible and safe operation that is associated with better operative and postoperative results and less pain. Various sizes of peripheral and central hydatid cysts can be removed safely.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Kamran Ali) for the series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.01.09). The series “Asia Thoracoscopic Surgery Education Program (ATEP) Special Issue on Inflammatory Thoracic Diseases” was commissioned by the editorial office without any funding or sponsorship. AT serves as an unpaid editorial board member of Journal of Visualized Surgery from Jan 2017 to Dec 2018. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Isitmangil T, Sebit S, Tunc H, et al. Clinical experience of surgical therapy in 207 patients with thoracic hydatidosis over a 12-year-period. Swiss Med Wkly 2002;132:548-52. [PubMed]
- Auldist AW, Blakelock R. Pulmonary hydatid disease. In: Parikh DH, Crabbe D, Auldist A, et al. editors. Pediatric Thoracic Surgery. London: Springer, 2009:161-7.
- Aytac A, Yurdakul Y, Ikizler C, et al. Pulmonary hydatid disease: report of 100 patients. Ann Thorac Surg 1977;23:145-51. [Crossref] [PubMed]
- Turna A, Yilmaz MA, Haciibrahimoğlu G, et al. Surgical treatment of pulmonary hydatid cysts: is capitonnage necessary? Ann Thorac Surg 2002;74:191-5. [Crossref] [PubMed]
- Doğan R, Yüksel M, Cetin G, et al. Surgical treatment of hydatid cysts of the lung: report on 1055 patients. Thorax 1989;44:192-9. [Crossref] [PubMed]
- Cetin G, Doğan R, Yüksel M, et al. Surgical treatment of bilateral hydatid disease of the lung via median sternotomy: experience in 60 consecutive patients. Thorac Cardiovasc Surg 1988;36:114-7. [Crossref] [PubMed]
- Sihoe AD, Yim AP. Video-assisted pulmonary resections. In: Patterson FG, Cooper JD, Deslauriers J. editors. Thoracic Surgery (3rd Edition). Philadelphia: Elsevier, 2008:970-88.
- Li WW, Lee TW, Lam SS, et al. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest 2002;122:584-9. [Crossref] [PubMed]
- Sihoe AD. The Evolution of VATS Lobectomy. In: Cardoso P. editor. Topics in Thoracic Surgery. Rijeka: Intech, 2011:181-210.
- Chou SH, Chuang IC, Huang MF, et al. Comparison of needlescopic and conventional video-assisted thoracic surgery for primary spontaneous pneumothorax. Minim Invasive Ther Allied Technol 2012;21:168-72. [Crossref] [PubMed]
- Rocco G, Khalil M, Jutley R. Uniportal video-assisted thoracoscopic surgery wedge lung biopsy in the diagnosis of interstitial lung diseases. J Thorac Cardiovasc Surg 2005;129:947-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Evolving from conventional video-assisted thoracoscopic lobectomy to uniportal: the story behind the evolution. J Thorac Dis 2014;6:S599-603. [PubMed]
- Becmeur F, Chaouachi B, Dhaoui R, et al. Video-assisted thoracic surgery of hydatid cysts of the lung in children. J Chir (Paris) 1994;131:541-3. [PubMed]
- Paterson HS, Blyth DF. Thoracoscopic evacuation of dead hydatid cyst. J Thorac Cardiovasc Surg 1996;111:1280-1. [Crossref] [PubMed]
- Parelkar SV, Gupta RK, Shah H, et al. Experience with video-assisted thoracoscopic removal of pulmonary hydatid cysts in children. J Pediatr Surg 2009;44:836-41. [Crossref] [PubMed]
- Chowbey PK, Shah S, Khullar R, et al. Minimal access surgery for hydatid cyst disease: laparoscopic, thoracoscopic, and retroperitoneoscopic approach. J Laparoendosc Adv Surg Tech A 2003;13:159-65. [Crossref] [PubMed]
- Alpay L, Lacin T, Atinkaya C, et al. Video-assisted thoracoscopic removal of pulmonary hydatid cysts. Eur J Cardiothorac Surg 2012;42:971-5. [Crossref] [PubMed]
- Findikcioglu A, Karadayi S, Kilic D, et al. Video-assisted thoracoscopic surgery to treat hydatid disease of the thorax in adults: is it feasible?. J Laparoendosc Adv Surg Tech A 2012;22:882-5. [Crossref] [PubMed]
- Alpay L, Lacin T, Ocakcioglu I, et al. Is Video-Assisted Thoracoscopic Surgery Adequate in Treatment of Pulmonary Hydatidosis? Ann Thorac Surg 2015;100:258-62. [Crossref] [PubMed]
- Mallick MS, Al-Qahtani A, Al-Saadi MM, et al. Thoracoscopic treatment of pulmonary hydatid cyst in a child. J Pediatr Surg 2005;40:e35-7. [Crossref] [PubMed]
- Ma J, Wang X, Mamatimin X, et al. Therapeutic evaluation of video-assisted thoracoscopic surgery versus open thoracotomy for pediatric pulmonary hydatid disease. J Cardiothorac Surg 2016;11:129. [Crossref] [PubMed]
- Ocakcioglu I, Sayir F. Uniportal Thoracoscopic Approach For Pulmonary Hydatid Cyst: Preliminary Results. Surg Laparosc Endosc Percutan Tech 2018;28:298-302. [PubMed]
- Eroglu A, Aydin Y, Altuntas B. Video-Assisted Thoracoscopic Surgery Is Safe and Effective in the Treatment of Pulmonary Hydatid Cyst. Ann Thorac Surg 2016;101:829. [Crossref] [PubMed]
- Bakhytzhan S, Mukhtar S, Ruslan K, Denis V, Gulziya I. Single-center experience in the surgical treatment of combined lung Echinococcosis. Saudi Med J 2018;39:31-7. [Crossref] [PubMed]
- Turna A. Aspiration of cystic content of a patient with bilateral pulmonary hydatid disease. Asvide 2019;6:034. Available online: http://www.asvide.com/article/view/29875
- Turna A. Removal of germinative membrane of the patient with right pulmonary hydatid disease. Asvide 2019;6:035. Available online: http://www.asvide.com/article/view/29877
Cite this article as: Turna A. Minimally invasive approach for pulmonary hydatid cyst. J Vis Surg 2019;5:16.