
Awake non-intubated pulmonary metastasectomy
IntroductionOther Section
Non-intubated awake thoracic surgery is gradually gaining more popularity and an increasing number of surgical procedures is carried out in many dedicated centers all over the world (1). Since the beginning of the experience the resection of peripheral pulmonary nodules has been widely considered one of the main indications for non-intubated thoracic surgery (2). In this setting surgery of lung metastases appears to be a quite interesting field of application. Actually, lung metastases are usually located in the peripheral parenchyma and so lying in a position suitable for resection through video-assisted thoracic surgery (VATS) (3).
Moreover, the success of uniportal VATS and the development of instruments specifically crafted for it made this surgery much more agreeable and feasible (4). Lastly, the introduction of new drugs and the development of new anesthesiological devices (5) convinced even many reluctant surgeons to exceed the psychological threshold of working in a “moving lung” operative field.
Based upon these observations, resection of isolated pulmonary metastases under non-intubated anesthesia has become a widely accepted treatment for appropriately selected patients (6). Hereby, we are glad to present our entire experience in lung metastasectomy under awake non-intubated anesthesia.
MethodsOther Section
Patient selection
Accurate selection is pivotal to correctly address patients to awake non-intubated lung metastasectomy. They must fit four categories that are synthetically described in Table 1. Mandatory requirements for lung metastasectomy still remain the control of the primary tumor as well as the resectability of all viable lung metastases (7). Some author has recently introduced the term of “primary tumor controllable” (8,9), implying the resection or radical cure of the primary tumor even after lung metastasectomy. Prediction of adequate postoperative respiratory function and quality of life is another pivotal prerequisite, but due to the small number of lesions considered treatable through non-intubated pattern should not be consider a potential hinder.
Table 1
| Prerequisites for lung metastasectomy |
| Treated or treatable primary tumor |
| Absence of extrapulmonary metastatic disease or, if present, controllable by surgery or multimodality therapies |
| Adequate cardiopulmonary post-resectional reserve |
| VATS feasibility |
| Anamnestic or imaging absence of pleural adhesion |
| Normal coagulation tests and absence of bleeding disorders |
| Non-obesity (body mass index <30 kg/m2) |
| Criteria for VATS metastasectomy |
| Oligometastases with possible long free-disease interval |
| Clinically negative mediastinal nodes |
| Peripheral location |
| No more than 3 cm in maximum diameter at computed tomography |
| Non-intubated procedure feasibility |
| Signed fully informed consent to awake non-intubated procedure |
| Easy accessibility to the airways |
| Stable and cooperative psychological profile |
VATS, video-assisted thoracic surgery.
Second point is the feasibility of a VATS procedure. In this sense a mandatory prerequisite is the absence of tenacious and/or diffuse pleuro-pulmonary adherences. Their presence can be predicted either on anamnestic or imaging (i.e., echography or computed tomography) bases. Furthermore, patients scheduled for VATS should not be at risk for bleeding this meaning coagulation tests within a physiological range and anamnestic exclusion of coagulopathies. Obesity is considered another major limitation to VATS and patients with a body mass index greater than 30 kg/m2 should be addressed to other surgical accesses.
Third criteria are the technical/oncological resectability of the metastasis through a VATS approach. This condition outlines a small, exiguous number (oligo) and peripheral metastasis, easy findable or palpable, removable with a limited resection, possibly with a long disease-free interval and with no clinical evidence of metastatic mediastinal nodes.
Last group of prerequisites concerns the specific practicability of the VATS through a non-intubated modality. These requirements entail the expressed patient’s will to undergo a non-intubated procedure, the cooperative and stable mood ascertained by a psychologist with a specific questionnaire: Profile of Mood States (10) and Mini Mental State Examination (11). Easy access to airways is the final need for a non-intubated procedure. This point may be very important whenever a fast-track conversion to intubated procedure is required.
All these conditions have to be collegially discussed and decided by a multidisciplinary committee compounded by surgeons, oncologist, radiotherapists, anesthesiologists, intensivists, pneumologists, radiologists and psychologists.
In all patients the diagnosis is normally suspected during follow-up after having performed routine total body computed tomography. However, in order to achieve the best decision for the patient, preoperatory workup should be integrated with total body positron emission tomography, blood gas analysis, global respiratory function assessment with postoperative prediction, echocardiography and cardiologic evaluation.
Anesthesia
Non-intubated anesthesia especially if performed under awake pattern implies a robust communication between surgical team and patient. Patient must be aware of the discomfort due to the open pneumothorax, during which they have to maintain a normal breath rate. Both the surgeon and the anesthesiologist should calmly talk to the patient, controlling the level of anesthesia and updating him/her about the progress of the procedure.
We have found of utility the diffusion of low-volume classic music in order to transmit a perception of a calm and professional environment, improving patients’ acceptance of the non-intubated procedure.
Anesthesiologist should stay close to the patient having optimal control of the airways, rapid set up for double lumen intubation and a bronchoscope readily available. Monitoring with electrocardiogram, pulse oxymeter, systemic and central venous blood pressure, body temperature, arterial line, and end-tidal is suggested. Timed control of blood gases at the various phases of the operation is mandatory especially in the case of procedures prolonging over 30 minutes.
Technique
Anesthesiological technique has evolved through the time, becoming progressively less invasive. Throughout our experience we have used different anesthesiological patterns gaining knowledge about the different techniques. At the beginning of our experience VATS metastasectomy were approached with the awake patient, thus necessitating the complete collaboration with him/her. Analgesia and motor block were obtained with thoracic epidural anesthesia at the T1 to T8 level (12). The thoracic epidural catheter was positioned at the T4 level and a solution of ropivacaine 0.5% and sufentanil 1.66 g/mL was continuously infused during the procedure. Premedication with intravenous midazolam was always administered. In addition, in order to avoid cough reflex, a 5-mL solution of 2% lidocaine was preoperatively aerosolized.
In the event of an unsatisfactory block, supplementary local anesthesia was provided by local injection of a 50% mixture of ropivacaine (7.5%) and bupivacaine (2%). Onset of anxiety or panic was antagonized by continuous infusion of propofol (0.5 mg/kg) without suppressing spontaneous breathing. At the end of the procedure thoracic epidural infusion was reduced to ropivacaine 0.16% and sufentanil 1 g/mL at 2 to 5 mL per hour to provide postoperative analgesia.
We obtained overall good results, but this kind of anesthesia exposed the patient to a series of side effects such as hypotension and urinary retention as well as potential risk as infections, hematomas and hemorrhages.
On these bases we preferred to shift to a simple intercostal block by administering separately lidocaine 2% (4 mg/kg) on the site of incision up to the parietal pleura and ropivacaine 7.5% (2 mg/kg) along the competent intercostal nerve. Internal maneuvers are usually well tolerated with intraoperative intravenous administration of drugs for anxiety reduction (midazolam 0.03–0.1 mg/kg) and pain control (remifentanil 15 µg/kg/min). We introduced bispectral index analysis in order to carefully increase and visualize the level of sedation, which was possible to be safely reached and maintained optimizing the use of intravenous propofol without suppressing spontaneous breathing.
Surgery
Equipment
We prefer to use a 10-mm high-definition video-thoracoscope with 30° angulation. In order to simplify the procedure, long-shafted and curved instruments specifically crafted for uniportal surgery along with more traditional tools (e.g., ring forceps and curved vascular clamps) are mandatory to encompass the lesion. Energy devices by ultrasound (Ultracision®, Ethicon) or electrothermal bipolar vessel sealing (Ligasure®, Covidien) are game-changers in case of pleuro-pulmonary adhesions or small parietal and parenchymal hemorrhages, being able to easily divide and seal, respectively. Either manual (Endo-GIA®, Covidien) or powered (Echelon FlexTM, Ethicon) linear stapler with articulation and triple agraphes line (ranging from 2.5 to 3.5 mm) for parenchyma can be used. The patient is awake during the procedure. Thus precision, rapidity and effectiveness of actions are mainstays in the operative room. In order to reduce the discomfort derived from a prolonged open pneumothorax, a chest drainage connected to a water seal system is kept ready to be inserted inside the chest wall.
Position
First of all, due to awake status the position should be comfortable and long-lasting sustainable. The patient is usually positioned in a lateral decubitus fashion with both arms moderately elevated and frontally abducted. Secondly, a proper lateral curvature of the patient is the key for avoiding interference due to pelvis and elbow, but this may have some conflict with the patient’s tolerance. The most ergonomic position for surgeon is usually in the ventral side of the patient, with the assistant on the same side but in a caudal position and the scrub nurse on the dorsal side facing the main operator. We usually set main image and light management hub in cranial position and backward, thus enabling both easy access to airways for the anesthesiologist and good visualization for the surgeon.
Incision
At the beginning we used to perform the procedures with the patient placed in a full lateral decubitus position and through a 3-flexible-thoracoscopic trocar access disposed according to baseball diamond view (13). This access entailed three different areas for finger exploration but a more difficult capability in performing bidigital palpation (Figure 1).
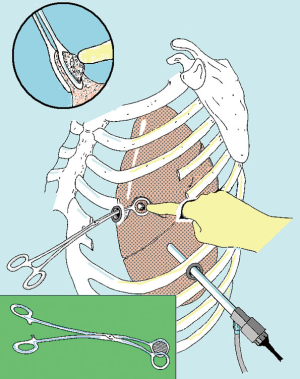
Lately, a 3–5 cm uniportal approach is generally preferred (13). The ideal incision can be performed according to the location of metastases and usually ranges between 4th and 6th intercostal space anteriorly to the margin of the latissimus dorsi, avoiding division of the muscle. However, skin incision should be adjusted at a distance convenient for finger exploration. An electrocautery is used to penetrate inside the aponeurosis in order to expose the serratus muscle, which is subsequently divided along the fiber’s direction. Once reached the chest plane is possible to coagulate the superior edge of rib limiting the chosen space and reach the pleural space with the diathermy, taking care not to injure the lung.
Initial incision is habitually extended with 1–2 finger insertion after having alerted the patient about possible discomfort about pneumothorax. Rib retraction is achieved by annular wound protector and portal spreader (Alexis®, Applied Medical, USA). The thoracoscope is always sited at the posterior end of the port in order to avoid instrument crowding (Figure 2). All these steps are usually well tolerated in the awake subjects and do not necessitate an additional supplementation of local anesthesia.
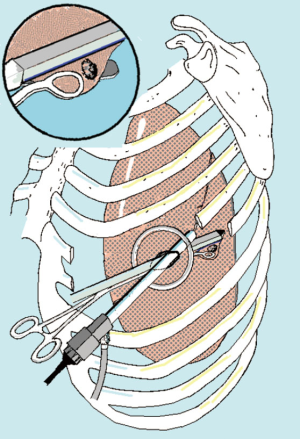
In alternative, especially in the case of bilateral lesions sited in the anterior segments of the lower lobes or in lingular or middle lobe, a subxiphoid incision can be taken into consideration. This is a 5-cm arciform-shape incision made one fingerbreadth below the costal arc. Each rectum abdominis muscle is divided along the linea alba and afterwards the pleural cavity opened just above the diaphragmatic anterior attachment. Even in this case the incision is protected with an Alexis retractor.
Palpation
The individuation of the targeted lesion is one of the most important action throughout the procedure. First of all, pleural cavity is explored and incidental adhesions freed by energy device. This action is important in order to obtain proper mobilization of the lung and, where possible, exteriorization of the lesion. At this time the presence of diffuse and tenacious adhesions should rapidly orientate towards the decision of continue the procedure under non-intubated pattern or shift to general anesthesia and open access. In the case of posterior-sited lesions the dissection of the pulmonary ligament can be easily performed by energy device after having infiltrated under vision the vagus nerve at hilum with 1-mL ropivacaine.
Preoperative assessment of the position by using sagittal, coronal and axial projections of the images at computed tomography is a fundamental step. We also found of utility to measure the gap between the metastasis and the closest fissure, since these relationships do not change very much within a partially collapsed lung. One should also consider that the metastasis is infrequently detectable at the mere visual exploration. Thus, palpation remains the easiest and fastest method for lesion individuation. This step is facilitated by the open pneumothorax, which reduces lung tissue density and make possible the identification of small nodules.
We recognize two types of palpation: direct by finger and instrumental by forceps. In doing awake metastasectomy we have to be sure that lesions can be reached by the finger and skin incision should be adjusted according to this purpose. Moreover, is possible to approximate the lung to the explorative finger or counter-push the parenchyma from the nearest fissure or from the basal or mediastinal faces depending on the site of the lesion. All these maneuvers can evoke cough leading to abrupt obliteration of the operative field. Thus, we experienced helpful the intraoperative infiltration along the phrenic nerve of 1–2 mL ropivacaine.
Sometimes is possible that even metastasis judged simple to be found can be neglected during initial palpation. In these cases, do not frustrate and keep palpating the lung looking at imaging. Palpation should be systematic following precise lines and planes without missing any presumable area. When the nodule remains impalpable do not hesitate to extend the incision after having removed Alexis retractor and supplemented infiltration of lidocaine, therefore permitting the exploration with two or more fingers. The enlargement should be preferentially performed by lengthening the anterior end of the wound given the greater breadth of the intercostal space. Notwithstanding all these actions can be straightforwardly performed in a well sedated patient and might significantly reduce of the operating time.
In case of lesions intraoperatively found not accessible to the finger, a ring forceps may be used by sliding and clamping. We have also developed a palpatory ring-disk forceps that reproduces bidigital-like full lung palpation (13) and provided good results in metastasis detection.
Although peripherally located, sometimes the research of the metastasis may be tedious and becoming the longer phase of the whole procedure. Therefore, intraoperative echography, image-guided preoperative wires or dye tracers already proposed for detecting pulmonary nodules in general anesthesia can be similarly adopted during non-intubated procedures.
Resection
When individuated the metastasis is then enclosed within bended endoscopic vascular clamps. This action helps to evaluate the tissue thickness and to facilitate the inclusion within the jaws of stapler. Either manual or powered linear stapler with a byte of 45–60 mm can be indifferently used in the same way as used under general anesthesia. Lung manipulation can easily elicitate cough reflex during stapling thus resulting in tears and bleeding. In order to avoid these problems, this step needs the most profound anesthesiologic level, which can be reached by using propofol or remifentanil. We found of help the suspension of the lesion either with a ring-forceps or by stitching for pulling the metastasis out of the wound. The exteriorization of the lesion makes the stapler positioning and firing much more easier. This is the reason why the choice of the skin incision and lung mobilization becomes very important steps in awake metastasectomy. After the resection the specimen is inspected in order to ascertain the presence of the lesion and to assess its morphologic appearance as well as the macroscopic distance from resection margins.
Closure
At the end of the procedure, a 28-Ch chest tube is placed at the posterior extremity of the wound. We do not routinely used intercostal sutures except longer incisions or in the case of emphysematous parenchyma, which might be a possible origin subcutaneous emphysema. Before tightening intramuscular stitches, lung re-expansion must be checked and can be more easily reached by inviting the patient to cough or by inserting for a while an aspirator inside the chest. For a better outcome of the procedure, the anesthesiologist should modulate the drug administration in order to allow the patient to achieve a normal status of consciousness and executing verbal orders.
Intubation
Intubation may be accomplished because of technical difficulties to maintain ventilation/sedation or surgical complications requiring open access. When occurring the need of intubation, the operation should be suspended closing the wound with a sterile adhesive drape, rotating the patient in supine position, which is more adapt to double lumen intubation. However, in the case of emergency intubation, intubation set must be kept ready and the anesthesiologist should be trained at double lumen intubation in a lateral decubitus. In alternative to avoid change of position we use to carried out an increasing number of non-intubated metastasectomies with the patient lying in semisupine decubitus.
Postoperative care
After a short stay in the recovery room under surveillance of the same anesthesiologist who performed the anesthesia during the procedure, the patient is sent to the ward. Drinking, eating and walking were usually restored in the same day of surgery. Similarly, early physiotherapy can be started at the bedside. The epidural catheter is removed 24 hours postoperatively.
ResultsOther Section
From 2000 to 2018, a total of 106 patients underwent lung metastasectomy with curative intent under non-intubated anesthesia. Fourteen patients had metastasectomy with a triportal and 92 with uniportal VATS, 10 of which through a subxiphoid approach.
Seven (6.6%) patients required the conversion to general anesthesia for intolerance of the procedure or technical difficulties. These patients were ruled out from the final statistical evaluation so reducing at 99 the total number of patients (12 multiportal and 87 uniportal). Clinical information and patient characteristics divided for multiportal and uniportal approach are listed in Table 2.
Table 2
| Variables | Total (n. pts =106) | Multiportal (n. pts =14) | Uniportal (n. pts =92) | P value |
|---|---|---|---|---|
| Age (years) | 64 [46–73] | 55 [49–67] | 66 [46–74] | 0.06 |
| Sex (M:F) | 56:50 | 8:6 | 48:44 | 0.7 |
| Need of intubation n (%) | 7 (6.6) | 2 (14.3) | 4 (4.3) | 0.06 |
| Histology* (carcinoma/sarcoma) | 73:26 | 9:3 | 64:23 | 0.5 |
| Previous chemotherapy (yes:no) | 63:43 | 10:4 | 53:39 | 0.1 |
| Disease-free interval* (months) | 22 [10–36] | 19 [12–26] | 23 [9–37] | 0.8 |
| Operative time* (min) | 31 [23–53] | 25.5 [23–33] | 32 [23–53] | 0.08 |
| Operating room stay* (min) | 51 [39–62] | 62.5 [55–70] | 47 [37–60] | 0.03 |
| Hospital stay* (days) | 3 [2–4] | 2.5 [2–3] | 3 [2–4] | 0.6 |
| Resected lesions* (number) | 147 | 18 | 129 | – |
| Number per patient (mean)* | 1.48 (147/99) | 1.50 (18/12) | 1.48 (129/87) | 0.9 |
| Volume of lesions (cm3)* | 4.0 [3.5–4.1] | 1.5 [1.0–1.7] | 4.1 [3.9–4.3] | 0.01 |
| Bilateral* | 8 | – | 8 | 0.3 |
| Metastases* | 120 | 15 | 105 | 0.8 |
| CT occult metastases* | 13 | 4 | 9 | 0.03 |
| Benign* | 27 | 3 | 24 | 0.8 |
| Mortality, n (%)* | – | – | – | – |
| Major morbidity, n (%)* | 6 (6.0) | 1 (8.3) | 5 (5.7) | 0.5 |
*, denominator excluding intubated patients. pts, patients.
Median operative time was 31 [interquartile range, 23–53] minutes and a global in-operatory room stay was 51 [39–62] minutes per procedure. Operative time resulted slightly shorter with triportal access, but global in-operating-room stay was significantly shorter (P=0.03) in uniportal group due to lesser anesthesia time (Table 2). Volume of metastases resected with uniportal approach resulted significantly greater (P=0.01). The median number of lesions resected per patient were 1.48 (Table 2) for a total of 129 nodules resected and 122 of which were predicted by CT scan. Median volume of the lesions was 4.1 [3.9–4.3] cm3, with 80.1% of the resected nodules were found to be metastatic at histologic examination. Anthracotic lymph node (n=11), granuloma (n=7) and hamartoma (n=6) were found in the remaining 19.9% of cases.
No mortality was documented. Major morbidity affected 6 patients (6.1%): 4 patients with persistent air leak, 1 pneumonia and 1 persistent arrhythmia. No differences were detectable for type of approach. Median drainage time was 14 [10–31] hours and total length of hospital stay was 3 [2–4] days. Not even in this case differences between group were evidenced.
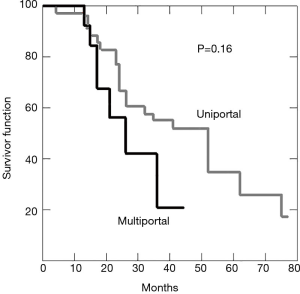
Seventy-eight (78.8%) patients recurred, 28 (35.9%) of which developed ipsilateral lung metastasis and 50 (64.1%) in the contralateral lung. Median time of recurrence was 15 months. Forty-two patients died of recurrence, 28 are still alive, 16 remained clinically tumor free. Another 29 died for other cause. Post-operative overall survival at 3 years was 49%. Kaplan-Meier curve for overall survival in represented in Figure 3.
DiscussionOther Section
Extensive experience with pulmonary metastasectomy in a number of different cancers has confirmed that resection can substantially prolong survival and cure some patients (14,15). Despite the lack of randomized trials, multiple case reports and small series suggest that resection prolongs survival and that long-term relapse-free survival (i.e., cure) is possible in some patients with isolated lung involvement.
Since 2000, we established the program named “Awake Thoracic Surgery Research” under the guidance of Prof. Tommaso Claudio Mineo (16). To our knowledge, this program is the first in the world to be created for this specific purpose. This investigational program included many interdisciplinary investigations aimed at understanding physiology and refining techniques and indications.
As previously described, at the beginning of our experience procedures were carried out in an awake patient under thoracic epidural anesthesia (12). This anesthesia was successfully applied to both nononcologic (pneumothorax, emphysema, pleural infection, pulmonary nodules, interstitial lung disease) and oncologic conditions (malignant pleural effusion, peripheral lung cancers, lung metastases, mediastinal masses) (16). The operation was carried out through a triportal access.
Thereafter, we shifted to a non-intubated technique entailing local anesthesia, vagal blockade and sedation assessed by bispectral index analysis (5) (Figure 4). The change from three to uniportal procedure facilitated the diffusion of this last technique (6,14). Many procedures are now possible through a single small incision, which reduces immunological and inflammatory response, morbidity, postoperative pain, hospital stay and global economic costs (18,19).

Comparing the two procedures we found that multiportal VATS had a shorter operating time but compensated by the longer time for establishing effective thoracic epidural anesthesia. We experienced that relatively longer operative time for uniportal procedure deemed in about 6 minutes more was mainly due to the research of the metastasis and to greater volume of metastasis considered for resection. Once again selection for awake non-intubated lung metastasectomy should include patients with peripheral oligometastases easy to palpate and small enough to be safely resected with a limited number of staple firing.
Major limitations are the exiguous number of multiportal procedures performed at the beginning of the learning curve that could represent an important bias. Furthermore, there is a very consistent interval spent between the two consecutive two techniques that makes the technical differences relevant.
In conclusion, we would like to take home the following messages. Surgical resection of lung oligometastases is a worthy procedure with a significant effect on long-term survival. Awake non-intubated lung metastasectomy is feasible but this choice implies correct selection of the disease and of the patient. We would state that the lesions likely predictable as difficult to palpate should not be considered suitable for awake surgery and should deserve general anesthesia. We think that this remains the main indication for non-intubated surgery.
AcknowledgmentsOther Section
During the editing of this paper Professor Tommaso Claudio Mineo, who inspired and initiated this study, suddenly died. The authors feel very grateful and deeply in debt to him.
Funding: None.
FootnoteOther Section
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michel Gonzalez) for the series “Advancement in the Surgical Treatment of Pulmonary Metastasis” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.03.11). The series “Advancement in the Surgical Treatment of Pulmonary Metastasis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This observational and retrospective study was approved by the ethics committee of our university (No. 016B/17). Individual informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Mineo TC, Ambrogi V. Efficacy of awake thoracic surgery. J Thorac Cardiovasc Surg 2012;143:249-50; author reply 250-1. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Dowling RD, Keenan RJ, Ferson PF, et al. Video-assisted thoracoscopic resection of pulmonary metastases. Ann Thorac Surg 1993;56:772-5. [Crossref] [PubMed]
- Migliore M, Deodato G. A single-trocar technique for minimally-invasive surgery of the chest. Surg Endosc 2001;15:899-901. [Crossref] [PubMed]
- Kissin I. Depth of anesthesia and bispectral index monitoring. Anesth Analg 2000;90:1114-7. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Fabbi E, et al. Uniportal non-intubated lung metastasectomy. J Vis Surg 2017;3:118. [Crossref] [PubMed]
- Ehrenhaft JL, Lawrence MS, Sensenig DM. Pulmonary resections for metastatic lesions. AMA Arch Surg 1958;77:606-12. [Crossref] [PubMed]
- Rusch VW. Pulmonary metastasectomy: a moving target. J Thorac Oncol 2010;5:S130-1. [Crossref] [PubMed]
- Nichols FC. Pulmonary metastasectomy: role of pulmonary metastasectomy and type of surgery. Curr Treat Options Oncol 2014;15:465-75. [Crossref] [PubMed]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services, 1971.
- Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry 1983;40:812. [Crossref] [PubMed]
- Mineo TC. Epidural anesthesia in awake thoracic surgery. Eur J Cardiothorac Surg 2007;32:13-9. [Crossref] [PubMed]
- Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg 2007;133:960-6. [Crossref] [PubMed]
- Ambrogi V, Sellitri F, Perroni G, et al. Uniportal video-assisted thoracic surgery colorectal lung metastasectomy in non-intubated anesthesia. J Thorac Dis 2017;9:254-61. [Crossref] [PubMed]
- Treasure T, Milošević M, Fiorentino F, et al. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014;69:946-9. [Crossref] [PubMed]
- Mineo TC, Tacconi F. From "awake" to "monitored anesthesia care" thoracic surgery: A 15 year evolution. Thorac Cancer 2014;5:1-13. [Crossref] [PubMed]
- Ambrogi V, Perroni G, Mineo TC. Non-intubated uniportal VATS lung metastasectomy. Asvide 2019;6:100. Available online: http://www.asvide.com/article/view/30986
- Mineo TC, Sellitri F, Vanni G, et al. Immunological and Inflammatory Impact of Non-Intubated Lung Metastasectomy. Int J Mol Sci 2017;18: [Crossref] [PubMed]
- Mineo TC, Tamburrini A, Perroni G, et al. 1000 cases of tubeless video-assisted thoracic surgery at the Rome Tor Vergata University. Future Oncol 2016;12:13-8. [Crossref] [PubMed]
Cite this article as: Ambrogi V, Perroni G, Mineo TC. Awake non-intubated pulmonary metastasectomy. J Vis Surg 2019;5:38.


