
Pursuit of an optimal surgical margin in pulmonary metastasectomy
Introduction
Pulmonary metastasectomy is an accepted part of the interdisciplinary treatment of stage IV disease in many different primary cancers and sarcomas. The treatment goal is either curative by complete removal of individual metastatic sites or prolonged survival by radical cytoreduction and hampering further tumor cell dissemination. In case of multiple pulmonary nodules, the objective of metastasectomy is not only to remove all gross tumors, but to preserve as much normal lung tissue as possible. This leads to a conflict between radicalism and tissue preservation. While the surgeon has no control on invisible micro-metastases, his responsibility is to completely remove visible or palpable tumor and prevent local intrapulmonary recurrence. As per the surgical recommendations, a conical-shaped wedge of pulmonary parenchyma is removed by cauterizing circumferentially around the nodule, taking with it a 0.5- to 1.0-cm margin of normal lung tissue in all directions (1). This was formulated on the basis of individual experience but without underlying scientific evidence. The intention of our study is to collect current evidence on surgical margins during pulmonary metastasectomy with focus on local recurrence prevention and parenchyma preservation.
Methods
To reach a consensus about the optimal surgical resection margin, below mentioned aspects need to undergo an in-detail analysis. First is the impact of risk factors and local recurrence on the disease course. Secondly, surgical technique and devices may influence local recurrence. Thirdly, the relationship between the molecular constellations and microscopic tumor spread in lung tissue with local recurrence. Lastly, the role played by the type of resection and status of intrathoracic lymph nodes. To this goal, we performed a literature review using keywords; “pulmonary metastasectomy”, “growth patterns”, “local recurrence”, “lymphatic involvement or lymph node involvement” and “safety margins”. In addition to this, the findings of our own research are included.
Results
Clinical experience on local recurrence
Local recurrence may occur as tumor growth at the resection line or in the draining hilar or mediastinal lymph nodes. Local recurrence at the former site can be related to completeness of resection and/or safety margin and the latter to the extent of lymph node dissection. Local recurrence in the lung is reported in 0.0% to 64% of patients after pulmonary metastasectomy (2-7). In a retrospective evaluation Shiono et al. reported local recurrences in 17 (28%) of 61 patients after wedge resection or segmentectomy for colorectal cancer (CRC) lung metastases (2), from these 15 cases already had a pathologic complete (R0) resection.
In 335 CRC lung metastases patients with 679 resections, there were 11.8% recurrences after 2 years and 20.6% after 5 years. Local recurrence was influenced by longer margins with a hazard ratio (HR) of 0.43 for every additional cm (P=0.015) and every additional cm increase in size of the metastasis was associated with an HR of 1.52 (P=0.012). Here the authors recommend a resection margin of at least half the tumor size to keep the rate of local recurrence <11% (5).
Evaluation of surgical margins with lavage cytology was described by Higashiyama et al. From resection margins in 87 pulmonary metastasectomies, 10 (11%) had positive cytological results despite a macroscopically safe margin. As a consequence, completion segmentectomy or laser evaporation of the margins was performed. No local recurrence was found when cytological results had been negative, but occurred in 2 with former positive cytological result (P<0.001) (8).
Surgical technique
Local recurrences rate of 28% was described after using staples for wedge resection or segmentectomy for the removal of CRC lung metastases and of the total 17 local recurrences, 2 were R1 and occurred in the staple line (2). A comparable rate of local recurrences was found in a Spanish multicenter comparison of minor resections (wedge or segment) with major resections (lobectomy or pneumonectomy). The local recurrence rates were 17.4% and 14.3% respectively. Altogether, in 42 of 522 patients (16.9%) recurrence at the resection line was encountered (9). Impact on incidence of local recurrence by employment of different resection techniques, such as electrocautery (211; 51.2%), laser (48; 11.7%), staples (119; 28.9%) and conventional wedge resection between clamps (34; 8.3%) was analyzed and published but no significant difference was found (P=0.318) (10).
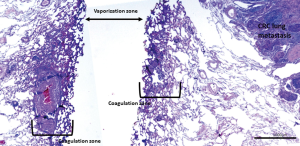
The Nd:YAG laser with a wavelength of 1,318 nm causes coagulation and shrinking of lung alveoli (Figure 1). In addition, the interstitial spaces, capillaries, small lymphatic and blood vessels are occluded by heat dispersion further avoiding tumor cell spread (11). No recurrences at the laser resection margin in 100 consecutive patients with follow-up of up to 5 years were reported by Rolle et al. (4). Others reported comparable results (6). Laser assisted surgery (LAS) was performed on 256 metastases in 99 patients, non-laser-assisted surgery (NLAS) on 127 metastases in 79 patients, and the 5-year survival rates were 69.3% in all patients, 65.7% after LAS and 73.6% after NLAS. There was no statistically significant survival difference after LAS or NLAS (P=0.41). The rate of local relapse was 0.8% after LAS vs. 3.1% after NLAS (P=0.073) (6) (Figure 2).

Microscopic tumor spread
Spread through air spaces (STAS) (P=0.04) and microscopic incomplete resection (P=0.02) have been significantly associated with local recurrence in surgery for CRC lung metastases. The distance of STAS tumor cells to the main tumor ranged from 0.5 to 10.0 mm (median, 2.0 mm). More STAS complexes increased the risk for recurrence (2). Furthermore STAS (P=0.02) and vascular invasion (P=0.02) were independent prognostic factors with a 5-year survival rate of 24.7% when STAS and vascular invasion was present and 93.3% when they both were negative (P=0.0002). In this patient cohort, a positive surgical margin (6%) was not associated with prognosis (P=0.77) (13).
STAS is well known in adenocarcinomas of the lung. Kadota et al. compared 291 lobectomies with 120 sublobar resections for small (≤2 cm) lung adenocarcinomas and found the risk of any recurrence being significantly higher in patients with STAS-positive tumors (42.6% vs. 10.9%; P<0.001 within 5 years) and its presence correlated with higher risk of distant (P=0.035) and locoregional recurrence (P=0.001). However, in the lobectomy group, the presence of STAS was not associated with any of the above-mentioned risks (P=0.50) (14).
Local spread of tumor cells beyond the border of the main metastases were analyzed in 102 sections of 17 CRC lung metastases by Welter et al. They found interstitial spread (41% cases), lymphatic spread (23% cases) and identified STAS and satellite tumor cells by immunohistochemistry in 16 of 17 metastases. The mean distance of satellite cells to the nodule was 0.99 mm (range, 0.06–6.43 mm). Furthermore, the probability of complete removal of satellite tumor cells was calculated to increase from 68.3% to 95.5%, and 99.7% with increasing distance of 1.59 to 3.43 mm, and 7.4 mm from the main metastasis, respectively. They concluded that tumor satellite cells might be the source of local recurrence when a safety distance during resection is less than 8 mm for larger and 3 mm for smaller lesions (15).
In a study of 183 patients with 459 metastasectomies, melanoma metastases had the highest incidence of lymphangitic spread [odds ratio (OR) 2.0 vs. sarcoma]. Whereas, sarcoma metastases had the highest incidence of pleural infiltration (OR 4.3 vs. CRC) and the lowest incidence of lymphangitic spread (3.5%). STAS were highest in CRC metastases (OR 29.3 vs. sarcoma) but was almost absent in sarcoma, melanoma and renal cell carcinoma metastases (<6%). Distinct invasion patterns exist for almost every primary tumor type and certain aggressive patterns of local growth (pleural infiltration, L1, V1, interstitial growth) increase in frequency by around 2.5% with every additional mm of the metastasis size. Metastases with microscopically smooth surface were less often associated with local tumor recurrence (P=0.088) (10).
In 52 sarcoma patients with 261 metastasectomies, interstitial growth (P=0.008) and metastasis >35 mm (P=0.023) had significant prognostic impact. While, pleural penetration (P=0.007) and metastasis >5 mm were risk factors for local intrapulmonary recurrence (16).
Lymphatic microvessel density (LMVD) and lymphovascular invasion (LVI) is another aspect of aggressive local tumor spread (Figure 3). Schweiger et al. found a significant association between increased LMVD or presence of LVI and an early tumor recurrence in locoregional lymph nodes and decreased overall survival (P<0.001) and (P=0.029) after pulmonary metastasectomy for CRC lung metastases (17). Recurrence at the resection site was unfortunately not evaluated.

Influence of mutational status
KRAS mutation in CRC was recently identified as a negative prognostic factor in right-sided colon cancers. Furthermore, KRAS-mutated colorectal liver metastases are more aggressive than KRAS wild-type tumors. In a retrospective study from Margonis et al. resection of liver metastases in KRAS wild type and KRAS mutated patients were analyzed. They observed a significant median disease-free survival difference in favor of anatomical resections in patients with a KRAS mutation (10.5 vs. 33.8 months; (P<0.001), whereas there was no difference in the group with KRAS wild type (P=0.142) (18). A comparable association for lung metastases was described by Renaud et al. in a retrospective study of 574 consecutive lung metastasesectomies for CRC secondaries they identified 168 patients with one isolated lung lesion and 95 (56.5%) of them harbored KRAS mutations (19). The type of resection did not impact the median OS in wild-type patients (P=0.67) but was significantly better in univariate and multivariate analysis following anatomical resection in KRAS patients (101 vs. 45 months, P=0.02). Additionally, the resection-margin recurrence rate was significantly higher for non-anatomical resections in KRAS patients (54.2% vs. 4.8%, P=0.001) in comparison with wild-type patients (P=0.97). The aggressive feature in the primary colon cancer is “tumor budding”, which is defined as individual cells or cell clusters of up to four or five cells at the invasive margin of CRC, protruding in the healthy tissue around. Its association with KRAS mutation is well known (20,21).
Anatomic mode of resection
Resection of lung metastases is performed as enucleation with cautery, bipolar scissors or lasers, wedge resection using staples or clamps, or anatomical resections like segmentectomy, lobectomy or pneumonectomy. A recent retrospective study from a Japanese nationwide CRC lung metastases database including patients from 2004–2008 (pre- targeted therapy era) evaluated recurrence patterns after wedge resection (N=455) or segmentectomy (N=98) (22). Apart from the larger median size of resected metastases in the segmentectomy group (18 mm) as compared to the wedge resection group (14 mm) (P<0.001), they were both comparable. An increased recurrence rate at the resection-margin after wedge resection when compared with segmentectomy (7.3% vs. 2.0%; P=0.035) was found. The multivariate analysis revealed that segmentectomy was a significant favorable factor for recurrence (HR: 0.63; P=0.005). This advantage of segmentectomy over wedge resection was, however, only demonstrated as a trend of overall survival (HR: 0.65; P=0.080) and did not take KRAS mutational status into account (22).
A retrospective analysis showing superiority of larger anatomical resection over wedge resection was presented by Hernández et al. In this a total of 522 patients were classified into major anatomic resections [lobectomy (N=100) and pneumonectomy (N=4); total 104 (19.9%) cases] and minor resections group [wedge resection (N=399) and segmentectomy (N=19), total 418 (80.1%) cases]. Both the univariate (P=0.03 and P<0.001) and multivariate (P=0.031 and P<0.001) analysis showed a better disease specific survival and a better disease-free survival in the major anatomic resections group. The rate of local and pulmonary metastatic recurrence was slightly higher in the minor resections group (17.4% and 24.9%) as compared with the major resection group (14.3% and 17.1%). However, the major anatomic resections group had a significantly larger metastases size than the minor resections group (P<0.001) (9).
Discussion
The goal of pulmonary metastasectomy is to completely remove tumor manifestations in the lung and intrathoracic lymph nodes. Unfortunately, local recurrence in the lung may occur, despite pathologic R0 resection. Triggering factors can be identified with hematoxylin & eosin staining (STAS, interstitial growth, satellite nodules, perivascular growth, L1, V1 and others), immunohistochemistry (isolated satellite cells, L1, tumor cells within inflammatory infiltrates, and others) or may by indirectly characterized with molecular pathology (KRAS mutation and others) (Table 1). These risk factors for local intrapulmonary recurrence can be identified in the resection specimen postoperative or may be calculated preoperative from the primary tumor (KRAS mutation, Grading, lymph node involvement). Radiomorphology of lung metastases to identify aggressive growth patterns in CT scans are currently under investigation. So understandably, the adequate safety margins are patient specific and are a result of a theoretical inclusion of all known and probable risk factors for recurrence (10).
Table 1
| Factor | Effect | Comments | P value | Reference |
|---|---|---|---|---|
| Surgical margin | Length (every additional increase in length of surgical margin) | HR 0.43 | 0.015 | (5) |
| Microscopic incomplete resection (increased incidence of local recurrence) | 0.02 | (2) | ||
| KRAS mutated CRC (non-anatomical resections have higher resection margin recurrence) | 0.001 | |||
| Metastasis size | Every additional cm increase in metastasis size | HR 1.52 | 0.012 | (5) |
| Laser assisted surgery | Lower local recurrence rates | (4,6) | ||
| STAS | Increased incidence of local recurrence | 0.04, <0.001 | (2,14) | |
| Absence increases 5 years survival | 0.0002 | (13) | ||
| Higher distant and locoregional recurrence | 0.035, 0.001 | (14) | ||
| Adversely affects prognosis | 0.02 | (13) | ||
| Highest in CRC metastases | OR 29.3 vs. sarcoma | (10) | ||
| Vascular invasion | Adversely affects prognosis | 0.02 | (13) | |
| Absence increases 5 years survival | 0.0002 | (13) | ||
| Pleural infiltration | Sarcoma vs. CRC | OR 4.3 vs. CRC | (10) | |
| Sarcoma | Interstitial growth | 0.008 | (16) | |
| Metastasis >35 mm (significant prognostic impact) | 0.023 | |||
| Pleural penetration (risk factor for local intrapulmonary recurrence) | 0.007 | |||
| Metastasis >5 mm (risk factor for local intrapulmonary recurrence) | ||||
| LMVD & LVI | Indicator of early tumor recurrence | <0.001 | (17) | |
| Decreased overall survival | 0.029 | |||
| KRAS mutated CRC | Longer median disease-free survival with anatomic resections | <0.001 | (18) | |
| Surgery | Segmentectomy (lower incidence of recurrence) | 0.63 (HR) | 0.005 | (22) |
| Wedge vs. segmentectomy (wedge has higher incidence of recurrence) | 0.035 | |||
| Major1vs. minor2 resections (major resections have better disease specific and better disease-free survival) | Univariate | (9) | ||
| Multivariate |
1, major resections, lobectomy & pneumonectomy; 2, minor resections, wedge resection & segmentectomy. HR, hazard ratio; KRAS, kirsten rat sarcoma oncogene mutation; CRC, colorectal carcinoma; STAS, spread through air spaces; OR, odds ratio; LMVD, lymphatic misrovessel density; LVI, lymphovascular invasion.
The optimal margin during pulmonary metastasectomy is a compromise between radical approach with wide margins and preservation of parenchyma and lung function especially when multiple nodules are present (Figure 4). From the oncologic point of view, the removal of a complete lobe is optimal to prevent local recurrence because this anatomical unit is ideally bounded by visceral pleura in all directions which prevents tumor cell dissemination into adjacent lobes. The pleura is a certain barrier against microscopic tumor spread. The superiority of lobectomy over minor resections concerning disease specific survival, disease free survival and the rate of local recurrences had been demonstrated in a national register (9). Because this was a retrospective analysis and patients undergoing lobectomy usually have central or larger tumors, further conclusions are difficult to draw. It can be concluded, that a lobectomy is best to prevent local recurrence but may lead to a relevant decrease of lung function.
Anatomic mode of resection
Concerning CRC lung metastases Shiono et al. demonstrated that segmentectomy is superior over wedge resection when metastases were larger than 1.4 cm (22). The number of local recurrences were (2.0% vs. 7.3%; P=0.035). Again, a clear conclusion is difficult because the position of the lesions (peripheral, intermediate or central) was not reported. At the least, it could be supposed that anatomical resection includes the removal of intrapulmonary lymph nodes and vessels, and therefore prevents local recurrence more effectively than wedge resection. These results suggest that anatomical resection is more likely to eliminate the spread of hidden hematogenous/lymphogenous metastasis of CRC in the same lobe and hilar lymph nodes (23). This theory is supported by the findings, that the probability for aggressive growth patterns at the surface of lung metastases rises with increasing size of the metastasis (10). It has been demonstrated that non-small cell lung cancer (NSCLC) lesions greater than 2 cm have an inferior disease-free survival when not removed with an anatomical resection (24).
The need for anatomical resection might not only be dominated by tumor size or tumor position, but might be influenced by the presence of driver mutations in the metastasis, as was presented by Renaud et al. They found a significant improvement in OS and resection margin recurrence in KRAS mutated CRC lung metastases when anatomical resection was performed instead of wedge resection or enucleation (19). This shows that the aggressiveness of the lesion needs to be considered for resection planning. Future studies might discover other mutations or constellations in CRC or in other metastases calling for anatomical resections.
Microscopic tumor spread
Local recurrence after pulmonary metastasectomy occurs in up to 28% of wedge resections. One reason for recurrence is inadequate safety margins especially when R1 resections are performed (2,9,10). Margins can be inadequate even when macroscopically complete resection was achieved. The macroscopically normal lung tissue remaining in the patient, as well as the normal tissue around the resected metastasis may contain tumor satellite cells which can be detected only microscopically or sometimes only with immunohistochemistry (2,15). STAS were found within distance of 5–10 mm around the lesion with a maximum number of cells within 2 mm distance. STAS was associated with local tumor recurrence in CRC metastases (2), as well as in small primary lung adenocarcinomas after limited resection (14). The probability to find STAS or satellite tumor cells around CRC lung metastases decreases with the distance and is less than 1% in 7.5 mm from the metastasis surface (15). STAS can be found in nearly all epithelial tumor types but are rare in melanoma and renal cell carcinoma (10). So, a safety margin of 3–5 mm for lesions up to 10 and 8 mm margins circular around epithelial tumors larger than 10 mm is reasonable.
Pleural infiltration and sometimes subpleural dissemination can be found more often in metastases from sarcoma. This aggressive pattern of local spread is associated with increased risk for local tumor recurrence (10,16). Therefore, one can conclude that visceral pleural attachment or involvement can be used as a signal for the surgeon to increase lateral margins when sarcoma metastases are present.
The manifestation of lymphangiogenesis in lung metastases from CRC has been described as a risk factor for early lymph node recurrence. LMVD and LVI was evaluated in 71 patients after pulmonary metastasectomy from CRC. LVI was evident in 46.5% and 58.6% of metastases and corresponding primary CRC, respectively. This leads to the assumption that LVI can be expected in the metastases, when present in the primary CRC. LVI in the metastasis was associated with early tumor recurrence in intrathoracic lymph nodes and a decreased overall survival (P<0.001 and P=0.029) (17). This fits well with the finding, that mediastinal recurrence might be prevented by meticulous lymph node removal. Performing metastasectomy without lymph node clearance might cause tumor recurrence in more than 20% of CRC patients (25). Furthermore, infiltrated lymph vessels serve as a connection between metastasis and regional lymph nodes. So, one could assume, that wedge resection should be followed by an increased rate of local recurrence. But, despite the fact that all patients were closely followed with CT-scans, pulmonary recurrence at the resection line was not reported by the authors (17). So, it remains unclear if LVI increases the rate of local recurrence in the lung. One possible conclusion is that a meticulous lymph node dissection seems reasonable when LVI is present in the metastasis or in the primary tumor. Only anatomical resection can guarantee complete removal of lymphatic structures. Therefore, LVI may serve as a reasonable justification to perform anatomical resection in case of a singular metastasis.
Clinical experience on local recurrence
The knowledge about local intrapulmonary recurrence at the resection site, indicating an inadequate resection margin during pulmonary metastasectomy is still inconclusive. The number of recurrences depends on the further treatment after metastasectomy, the definition of local recurrence, the follow-up period and the mode of follow-up examination. The application of postsurgical chemotherapy may prevent local recurrence or may prolong recurrence free survival. That means that in some patients’ inadequate margins may not lead to local intrapulmonary recurrence during life-time. Many studies evaluated intrapulmonary versus extrapulmonary (distant) tumor recurrence. A differentiation between new metastases in the lung and recurrence at the resection margin is often not reported; therefore, these studies do not increase clinical knowledge concerning local recurrence and margins (7,17). Furthermore, the interpretation of local recurrence on follow-up CT scan may be misleading because cautery or laser resection can induce local inflammatory reactions and scar formation that resemble tumor recurrence. To our understanding, the definition of local intrapulmonary recurrence at the resection line must include CT-criteria and histological criteria. The CT-criteria should be increasing size of a lung nodule in a minimum of two separate CT evaluations, presence of scar formation between nodule and visceral pleura as residual from prior surgery, nodule in the same segment of former metastasis removal, metal remnants in the lesion after wedge resection with staples or intrapulmonary markings with clips. Histologic criteria after repeated metastasectomy should be: tumor around a staple line, identical tumor attached to, or within a scar formation. With this we can conclude that only repeated metastasectomies with proven histologic criteria of local recurrence or definitive evaluation of two follow-up CT-scans with a growing lesion in the resection scar can be used to increase clinical knowledge. Histologic verification by bronchoscopy or fine needle histology with the radiologic criteria of local recurrence may also lead to a clear-cut definition of local recurrence.
Influence of mutational status
Driver mutations like KRAS in CRC are well investigated. Recently, KRAS positive tumors were correlated with high-degree tumor budding and podia formation, indicating a certain pattern of local spread that looks more aggressive than other non-mutated adenocarcinomas of the intestinal tract (20). The evaluation of KRAS positive liver metastases demonstrated that segmentectomy was superior over wedge resection with respect of local recurrence (18) and this conclusion concerning CRC lung metastases was supported by Renaud et al. (19). All these results point out that KRAS mutated CRC grows more aggressively, infiltrates the surrounding tissue and causes local recurrence when non-anatomical resection is performed. It is difficult to draw conclusions about the size of safety margins or the direction of greatest necessary margin (central or lateral) but the current guidelines about safety margins seem to be inadequate. Other mutations, as far as we know, have not yet been evaluated with respect of local recurrence in lung surgery.
Conclusions
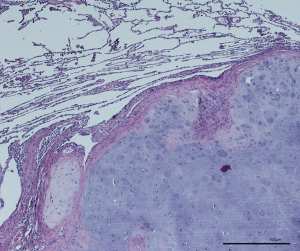
Safety margins prevent local intrapulmonary recurrence after metastasectomy. Wider safety margins are required when aggressive patterns of local spread increase the risk of local recurrence. Safety margins should be at least either half the diameter of the metastasis or 8 mm in case or CRC lesions that are 12 mm or more. To calculate the size of margins after laser enucleation, evaporation and coagulation zones on both sides of the resection line can be added to the pathologic measurement (Figure 2). Since the aggressiveness of a tumor depends on its histological and mutational status, whether or not an anatomical resection would be beneficial in cases of aggressive tumors need further research. Unfortunately to date, microscopic tumor dissemination can only be identified postoperatively. Studies are required to further describe the Radiomorphology and microscopic growth patterns of metastases, to facilitate surgeons to plan better and adequate resection margins.
Acknowledgments
We thank Ka-Won Noh for her great support in formatting and design of the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michel Gonzalez) for the series “Advancement in the Surgical Treatment of Pulmonary Metastasis” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.03.12). The series “Advancement in the Surgical Treatment of Pulmonary Metastasis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rusch VW. Pulmonary metastasectomy: current indications. Chest 1995;107:322S-31S. [Crossref] [PubMed]
- Shiono S, Ishii G, Nagai K, et al. Predictive factors for local recurrence of resected colorectal lung metastases. Ann Thorac Surg 2005;80:1040-5. [Crossref] [PubMed]
- Schmid S, Le UT, Zeisel C, et al. Pulmonary metastasectomy in sarcoma—experiences with laser-assisted resection. J Thorac Dis 2018;10:314-20. [Crossref] [PubMed]
- Rolle A, Koch R, Alpard SK, et al. Lobe-sparing resection of multiple pulmonary metastases with a new 1318-nm Nd: YAG laser—first 100 patients. Ann Thorac Surg 2002;74:865-9. [Crossref] [PubMed]
- Nelson DB, Tayob N, Mitchell KG, et al. Surgical margins and risk of local recurrence after wedge resection of colorectal pulmonary metastases. J Thorac Cardiovasc Surg 2019;157:1648-55. [PubMed]
- Franzke K, Natanov R, Zinne N, et al. Pulmonary metastasectomy–A retrospective comparison of surgical outcomes after laser-assisted and conventional resection. Eur J Surg Oncol 2017;43:1357-64. [Crossref] [PubMed]
- Chudgar NP, Brennan MF, Tan KS, et al. Is repeat pulmonary metastasectomy indicated for soft tissue sarcoma? Ann Thorac Surg 2017;104:1837-45. [Crossref] [PubMed]
- Higashiyama M, Kodama K, Takami K, et al. Intraoperative lavage cytologic analysis of surgical margins as a predictor of local recurrence in pulmonary metastasectomy. Arch Surg 2002;137:469-74. [Crossref] [PubMed]
- Hernández J, Molins L, Fibla JJ, et al. Role of major resection in pulmonary metastasectomy for colorectal cancer in the Spanish prospective multicenter study (GECMP-CCR). Ann Oncol 2016;27:850-5. [Crossref] [PubMed]
- Welter S, Arfanis E, Christoph D, et al. Growth patterns of pulmonary metastases: should we adjust resection techniques to primary histology and size? Eur J Cardiothorac Surg 2017;52:39-46. [Crossref] [PubMed]
- Venuta F, Rolle A, Anile M, et al. Techniques used in lung metastasectomy. J Thorac Oncol 2010;5:S145-50. [Crossref] [PubMed]
- Welter S, la Raja RB, Gupta V. A laser (Limax®, diode-pumped Nd:YAG laser, KLS Martin, Freiburg, Germany) resection of a sarcoma metastasis of the right lower lobe. Asvide 2019;6:101. Available online: http://www.asvide.com/article/view/30992
- Shiono S, Ishii G, Nagai K, et al. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg 2005;79:278-82; discussion 283. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Welter S, Theegarten D, Trarbach T, et al. Safety distance in the resection of colorectal lung metastases: a prospective evaluation of satellite tumor cells with immunohistochemistry. J Thorac Cardiovasc Surg 2011;141:1218-22. [Crossref] [PubMed]
- Welter S, Grabellus F, Bauer S, et al. Growth patterns of lung metastases from sarcoma: prognostic and surgical implications from histology. Interact Cardiovasc Thorac Surg 2012;15:612-7. [Crossref] [PubMed]
- Schweiger T, Nikolowsky C, Graeter T, et al. Increased lymphangiogenesis in lung metastases from colorectal cancer is associated with early lymph node recurrence and decreased overall survival. Clin Exp Metastasis 2016;33:133-41. [Crossref] [PubMed]
- Margonis GA, Buettner S, Andreatos N, et al. Anatomical resections improve disease-free survival in patients with KRAS-mutated colorectal liver metastases. Ann Surg 2017;266:641-9. [Crossref] [PubMed]
- Renaud S, Seitlinger J, Lawati YA, et al. Anatomical Resections Improve Survival Following Lung Metastasectomy of Colorectal Cancer Harboring KRAS Mutations. Ann Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Jang S, Hong M, Shin MK, et al. KRAS and PIK3CA mutations in colorectal adenocarcinomas correlate with aggressive histological features and behavior. Hum Pathol 2017;65:21-30. [Crossref] [PubMed]
- Prall F, Ostwald C. High-degree tumor budding and podia-formation in sporadic colorectal carcinomas with K-ras gene mutations. Hum Pathol 2007;38:1696-702. [Crossref] [PubMed]
- Shiono S, Okumura T, Boku N, et al. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg 2017;51:504-10. [PubMed]
- Perentes JY, Zellweger M, Gonzalez M. Personalized surgery for the management of pulmonary metastasis. J Thorac Dis 2018;10:52-5. [Crossref] [PubMed]
- Okada M, Nishio W, Sakamoto T, et al. Effect of tumor size on prognosis in patients with non–small cell lung cancer: the role of segmentectomy as a type of lesser resection. J Thorac Cardiovasc Surg 2005;129:87-93. [Crossref] [PubMed]
- Ali K, Cho S, Jang HJ, et al. Predictive Factors of Thoracic Lymph Node Metastasis Accompanying Pulmonary Metastasis from Colorectal Cancer. Thorac Cardiovasc Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Welter S, Barile La Raia R, Gupta V. Pursuit of an optimal surgical margin in pulmonary metastasectomy. J Vis Surg 2019;5:39.


