Robotic-assisted McKeown esophagectomy
Introduction
The incidence of esophageal cancer has rapidly increased in the last several decades, especially in the United States (1). Esophagectomy with lymph node dissection with or without neoadjuvant chemoradiotherapy is the standard treatment for early stage and locally advanced esophageal cancer. Despite significant improvement in the mortality rates, esophagectomy still carries significant morbidity.
Different surgical approaches of esophagectomy have been traditionally described. The three-stage or three-field esophagectomy was first described by K.C. McKeown in 1972 in England, and combined a laparotomy, right thoracotomy and cervical dissection and anastomosis (2). The McKeown (MKE) approach allows for a more comprehensive lymph node dissection and avoids the morbidity associated with an intra-thoracic anastomotic leak (3). In addition, the 3-field approach allows for better visualization of the intra-thoracic esophagus, although a thoracotomy can lead to increased pulmonary complications, especially in patients who underwent neoadjuvant therapy. With the hopes of decreasing the complication rate of such a morbid procedure, different minimally invasive approaches were developed including a robotic-assisted one, which was first described by Melvin et al. in 2002 (4).
The advantages of robotic surgery are multiple. The robotic platform provides superior visualization and more degrees of freedom; it also requires a lower learning curve compared to laparoscopic or video-assisted techniques. Multiple studies have in fact shown that robotic-assisted minimally invasive McKeown esophagectomy (McKeown RAMIE) is safe and provides similar oncologic outcomes in patients with esophageal cancer. For instance, Park et al. recently published a series of 114 patients who underwent robotic-assisted esophagectomy with a cervical anastomosis, achieving a R0 resection in 97.4% of patients. The authors demonstrated better outcomes in terms of left recurrent laryngeal nerve (RLN) lymph node chain dissection compared to VATS esophagectomy (5). On average they were able to dissect at least 10.8 lymph nodes in the RLN distribution group and showed that the learning curve for a robotic esophagectomy is about 20 cases, after which the rate of vocal cord paralysis decreases significantly. Another non randomized prospective study by Suda et al. demonstrated that robotic-assisted lymphadenectomy significantly reduced the incidence of vocal cord palsy, hoarseness and time on the ventilator (6).
Pre-operative evaluation/considerations
A thorough and extensive pre-operative evaluation of any patient undergoing esophagectomy is mandatory. The details of this evaluation are not discussed in this chapter. On the day of surgery, the patient is brought to the operating room and undergoes general anesthesia and endotracheal intubation. Either a single lumen tube with bronchial blocker or double lumen tube can be used for lung isolation. The patient is kept in the supine position and an esophagogastroduodenoscopy is performed to evaluate extent of tumor and exact location in the esophagus. This is specifically done to rule out extensive gastric involvement so that a gastric conduit can still be used, and to evaluate the proximal involvement. A nasogastric tube is placed in the stomach. Flexible bronchoscopy can also be performed to rule out involvement of the airway and to clear out secretions. A Foley catheter should be placed due to the length of procedure.
Surgical technique
Thoracic portion—right robotic-assisted thoracic surgery (RATS)
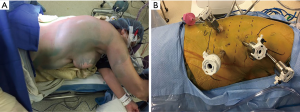
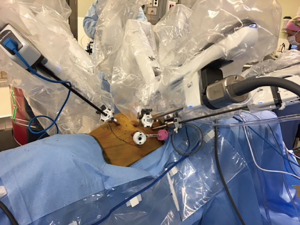
The patient is then repositioned in the left lateral decubitus position with flexion and 45° anterior tilting in a semi-prone position (Figure 1A). We begin the procedure by placing four 8-mm robotic ports (Figure 1B). The first port is the assistant port and is placed at the anterior axillary line in the 7th or 8th intercostal space (ICS). The remaining ports are placed under direct visualization using a 5 mm 30-degree thoracoscope. They are placed in the mid-axillary line in the 3rd and 6th ICS; the 3rd port is placed below the tip of the scapula, in about 9th ICS. The right hemi-thorax is insufflated with CO2 to a pressure of 8–10 mmHg and the anesthesia team isolates the right lung. The Xi da Vinci robot (Sunnyvale, CA, USA) is then docked. The 30 degrees camera is placed in the middle port, vessel sealer in the right arm and a fenestrated bipolar or Cadiere forcep in the left arm (Figure 2).
Dissection begins by dividing the inferior pulmonary ligament. This allows the lung to be retracted superiorly and anteriorly. The mediastinal pleura is then divided longitudinally to mobilize the esophagus up to the level of the azygos vein. The esophagus is then encircled with a Penrose drain, which helps with retraction as it is further mobilized cephalad above the level of the azygos vein, which is generally left intact. The azygos can be divided in case of a large mid-esophageal tumor. The vagus nerve is then divided bilaterally below the level of the RLN take-off. Both hemostasis and dissection are facilitated using the vessel sealer device. All of the lymph nodes in stations 7, 8, 9 and 2–4 as well as all of the peri-esophageal lymph nodes are dissected and removed. An additional Penrose drain is then placed around the distal esophagus. Both Penrose drains are left in the chest at the diaphragmatic hiatus and thoracic inlet to allow for dissection of esophagus from the abdomen and retrieval of the specimen from the left neck respectively (Figure 3). A 24 F Blake drain is left in the right chest along the posterior gutter, the robot is un-docked and all incisions closed in the standard fashion using 3-0 absorbable suture or staples (Figure 4). The bronchial blocker is removed and the robot is undocked without breaking sterility of instruments.

Cervical portion: cervicotomy
The patient is re-positioned in the supine position. A soft gel roll is placed under the patient’s left flank and shoulder. The head is turned to the right and the chest abdomen and neck are then prepped and draped. A left cervicotomy is performed along the anterior border of the sternocleidomastoid muscle similar to the incision used for carotid endarterectomy. The carotid sheath is retracted laterally and the pre-vertebral plane is developed. The Penrose drain is grasped and delivered into the wound (Figure 5). This facilitates exposure of the cervical esophagus, which is then dissected further, avoiding injury to the left RLN.
Abdominal portion: robotic-assisted laparoscopic surgery (RALS)
The left neck wound is packed and the abdominal portion of the operation is begun. We prefer abdominal entry using a Veress needle at the umbilicus and optical entry in the supra-umbilical position using a 5 mm trocar. The abdomen is insufflated to 15 mmHg and a standard laparoscope is used for the placement of the remaining robotic ports and liver retractor (Figure 6). The camera port is placed 1 hand breath away from the supra-umbilical position in the left paramedian position. The right- and left-hand ports are placed 1 hand breath away from the camera port in the right and left mid clavicular line. The retraction port is placed maximally laterally in the left flank a few centimeters below the costal margin. The supra-umbilical 5 mm port is exchanged for a 12 mm port and functions as the assistant port. A 5 mm port is placed separately in the right flank a few centimeters below the costal margin for the liver retractor. The liver retractor is mounted to the bed. The patient is positioned in deep reverse Trendelenburg position and the robot is docked once again. A tip-up fenestrated grasper is placed in the left most lateral port, a vessel sealer is used in the right arm and a fenestrated bipolar or Cadiere forcep is used in the left arm as the assistance instrument.
The goal of the abdominal portion of the procedure is to mobilize and create a tension free gastric conduit. Dissection is begun at the level of the pars flaccida and the gastro-hepatic ligament is divided. Dissection is then carried towards the diaphragmatic hiatus but the chest is not entered early on during the procedure as this would evacuate some of the pneumoperitoneum and make the rest of the procedure more difficult. The gastrocolic ligament is then divided alongside the greater curvature of the stomach, dividing all of the short gastric vessels. The gastroepiploic pedicle is identified and the greater omentum is divided towards the pylorus, taking great care to preserve the gastroepiploic artery at its take-off from the gastroduodenal artery.
The hepatic flexure portion of the greater omentum is then divided, further exposing the duodenum. We then perform an appropriate kocherization by dividing the lateral retroperitoneal attachments of the duodenum. The pylorus should reach the diaphragmatic hiatus in a tension free manner. We then replace the vessel sealer in the right arm with a bipolar Maryland forceps to create the pyloromyotomy. A 2-0 silk suture is placed in the pyloric muscle and the Maryland grasper is used to divide the muscle fibers overlying the suture. The muscle fibers should be divided down to the submucosal plane. The myotomy can be closed in a transverse fashion (Figure 7).

The stomach is then retracted superiorly and the retro-gastric attachments are taken down. The left gastric pedicle is identified and dissected free. A nodal dissection is carried out mobilizing the lymphatic tissue along the celiac artery towards the esophagus. The left gastric artery and pedicle are then divided using a linear endoscopic stapler. The phreno-esophageal ligament is then dissected exposing the lower Penrose drain. This ensures circumferential dissection of the esophagus at the level of the hiatus. At this point, the nasogastric tube is withdrawn into the thoracic esophagus and it is imperative to check with the anesthesia team that no other devices or tubes are left in the esophagus prior to creation of the conduit.
The conduit is created using a series of linear stapler loads and begins at the incisura and running along the greater curve to create a long 5 cm tube. Care must be taken during the creation of the conduit so that the posterior redundant wall of the stomach is not folded on itself. After the conduit is completed, it is sutured to the specimen using silk suture.
The assistant then provides gentle traction on the cervical esophagus and Penrose to pull the specimen and conduit up into the cervical wound. This must be done carefully under robotic vision to ensure that the conduit does not twist and is not under tension. Once the specimen and conduit have been delivered in the neck, the diaphragmatic hiatus can be closed robotically around the conduit to prevent herniation of abdominal contents into the thorax. At this point, a jejunal feeding tube can be placed with the use of the robotic platform; this portion of the procedure is discussed elsewhere. The robot is then undocked, liver retractor removed and the fascia at the supra-umbilical site is closed using an absorbable suture. All remaining robotic incisions are closed using suture or staples and attention is drawn towards the cervical esophagus.
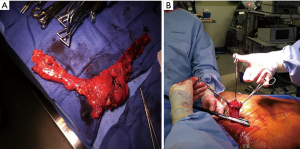
In the neck, the conduit is separated from the specimen by cutting the previously placed silk stitch. The nasogastric tube is pulled back into the oropharynx. The esophagus is divided proximally and a proximal margin is sent to pathology separately (Figure 8A). We then perform a side-to-side functional end-to-end anastomosis using a linear stapler (Figure 8B). The nasogastric tube is re-positioned distal to the anastomosis and the common enterotomy is also closed using a linear stapler. The anastomosis is then delivered back into the neck and a Jackson-Pratt drain is left in the wound, which is then loosely closed in 2 layers. The patient is then usually extubated and transferred to the recovery room then to the step-down unit.
Post-operative care
The patient is placed in a telemetry-monitored bed and the neo-esophagus is decompressed with a nasogastric tube, which is generally kept on suction for 4–7 days. Feeding via the jejunostomy tube is begun on post-operative day 2. The chest drain is closely monitored and removed when the patient is tolerating tube feeds at goal with no evidence of chyle or other suspicious leakage, and when the output is less than 250 cc per day. The patient is discharged home with a neck drain, which is taken out in the office. The patient’s diet is slowly advanced as an outpatient if a water-soluble contrast swallow study shows no leak or other abnormality; that is generally performed on post-operative days 10–14. Patients are encouraged to eat frequent small meals, sleep with the head of the bed elevated, avoid eating immediately before going to sleep while staying on proton pump inhibitors twice daily.
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA006927.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Robotic Surgery for Esophageal Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.03.14). The series “Robotic Surgery for Esophageal Cancer” was commissioned by the editorial office without any funding or sponsorship. AA served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Visualized Surgery from Jul 2018 to Jun 2020. RP reports personal fees from Veran Medical, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Patel N, Benipal B. Incidence of Esophageal Cancer in the United States from 2001-2015: A United States Cancer Statistics Analysis of 50 States. Cureus 2018;10:e3709 [PubMed]
- McKeown KC. Trends in oesophageal resection for carcinoma with special reference to total oesophagectomy. Ann R Coll Surg Engl 1972;51:213-39. [PubMed]
- D'Amico TA. Mckeown esophagogastrectomy. J Thorac Dis 2014;6:S322-4. [PubMed]
- Melvin WS, Needleman BJ, Krause KR, et al. Computer-enhanced robotic telesurgery. Initial experience in foregut surgery. Surg Endosc 2002;16:1790-2. [Crossref] [PubMed]
- Park SY, Kim DJ, Kang DR, et al. Learning curve for robotic esophagectomy and dissection of bilateral recurrent laryngeal nerve nodes for esophageal cancer. Dis Esophagus 2017;30:1-9. [Crossref] [PubMed]
- Suda K, Ishida Y, Kawamura Y, et al. Robot-assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: technical report and short-term outcomes. World J Surg 2012;36:1608-16. [Crossref] [PubMed]
- Patel S, Petrov R, Abbas A, et al. Thoracic dissection. Asvide 2019;6:111. Available online: http://www.asvide.com/article/view/31084
- Patel S, Petrov R, Abbas A, et al. Pyloromyotomy. Asvide 2019;6:112. Available online: http://www.asvide.com/article/view/31085
Cite this article as: Patel S, Petrov R, Abbas A, Bakhos C. Robotic-assisted McKeown esophagectomy. J Vis Surg 2019;5:43.