Robotic substernal esophageal bypass and reconstruction with gastric conduit—frequently overlooked minimally invasive option
Overview
Esophagectomy is a complex surgical procedure most commonly performed for malignancy and sparingly for end-stage benign esophageal diseases. First esophagectomy have been performed and reported in 1913 by Franz Torek in New York (1). Since that initial experience numerous modifications of the procedure have been introduced into clinical practice. These procedures vary by the type of the surgical access, the extent of the resection, level of the anastomosis, type of the conduit and the placement route. Modern esophagectomy includes immediate reconstruction of the gastrointestinal tract continuity after resection of the esophagus. Recently popularized minimally invasive and robotic esophagectomy mimic standard well-established procedures, but convey improved outcomes due to decrease in morbidity and mortality of the esophagectomy (2-4).
Immediate reconstruction after esophagectomy most commonly utilizes posterior mediastinal (native) route for the placement of the conduit. This is currently a preferred route for the conduit placement after esophagectomy. Despite decades-old conflicting data on the difference of the outcomes and the length of the route it has now been settled in all major esophageal centers (5). Previous anatomic studies demonstrated this route to be the shortest distance between abdomen and neck, requiring lesser length of the conduit for the reconstruction, potentially resulting in fewer complications (6,7).
The posterior mediastinal route has more normal, pseudo anatomical appearance. Perceived benefits of it are shorter distance and more normal anatomic appearance of the reconstruction, lesser incidence of the complications and lower mortality, better functional and cosmetic results and of course avoidance of additional filed of surgical dissection for the extraanatomic placement (5,6,8-11). Despite that, it has some disadvantages. For example, there is an increased risk of conduit obstruction and recurrent dysphagia in cases of incomplete resection or local recurrence (12). On the contrary, retrosternal route potentially offers advantages of avoiding dysphagia in these circumstances, spares conduit from the radiation field, avoids development of hiatal/paraconduit hernia and is a route of choice for staged or delayed reconstruction (11,13).
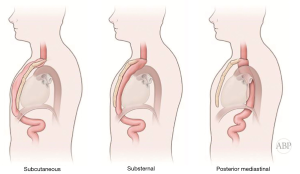
Multitude of studies have demonstrated posterior mediastinal being shorter then retrosternal route (14,15). However, several publications have disputed that data (7,16,17). Substernal route results in two significant angulations of the conduit on its course—one at the cervical level, where the esophageal stump is brought from prevertebral position through the thoracic inlet to the superficial retrosternal position. Second bend occurs at the level of the diaphragm, where the conduit again deviates posteriorly to reach the stomach, pylorus and eventually retroperitoneal location of the duodenum (Figure 1). These angulations can potentially impair transit of the food bolus in already aperistaltic conduit, contributing to swallowing difficulties and dysphagia and can potentially complicate endoscopy and dilations postoperatively (10,18). In addition, previous sternotomy with open heart surgery significantly complicates dissection and conduit placement. Retrosternal conduit in turn will compromise surgical access in cases of cardiac procedure in the future (19-21). For these reasons routine retrosternal conduit placement is currently avoided (9).
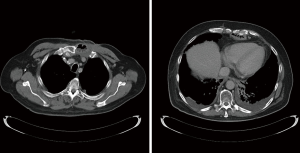
Occasionally, extraanatomic route is required for reconstruction of the esophageal continuity. Multitude of the extra-anatomic routes have been described, such as retrosternal (substernal), presternal (subcutaneous), transpleural (retro- and antehilar) (Figure 1). Transpleural routes are essentially modification of the posterior mediastinal bed with violation of the mediastinal pleura. With wide opening of the mediastinal pleura in Ivor Lewis and McKeown modifications, conduit is essentially prolapsing into the right pleural space (Figure 2). Presternal/subcutaneous route confers poor cosmetic results, leads to development of epigastric incisional hernia and poor functional outcomes and currently is rarely used (22,23). It has, however, been advocated as the choice of reconstruction in frail patients, sometimes as the two staged approaches (24,25). Out of the multitude of the extraanatomic routes for esophageal reconstruction, the largest experience is accumulated with retrosternal placement of the conduit (9,12,26-31).
Use of extra anatomic route can be justified in cases of unsuitable native esophageal bed for conduit placement. This is frequently the case in delayed reconstruction. Esophageal exclusion with the diverting esophagectomy can be performed as a salvage procedure for the control of mediastinal contamination in cases of uncontrolled esophageal leak, esophageal perforation or conduit, unusable for the reconstruction (9,32-34). This devastating scenario can be a complication of the previous esophageal surgery. Conduit necrosis with anastomotic leak after esophagectomy, leak after procedures for the benign foregut problems, such as paraesophageal hernia, achalasia and others can demand esophageal diversion, once conservative therapy and all endoscopic interventions to control the leak ultimately fail. This also might be required in cases of esophageal perforation, iatrogenic or spontaneous, especially with delayed presentation. Patients, surviving this devastating complication are desperately seeking restoration of the esophageal continuity for resumption of the oral intake and quality of life improvement (32,35,36).
Multiple studies demonstrated increased rates of complications and postoperative mortality in patients, undergoing retrosternal reconstruction after esophagectomy (5,8,17,18,29,37-39). However, in metanalysis there was no difference in outcomes (31). Some experts propose delay in the anastomosis creation after delivering gastric conduit to the neck by several weeks. In delayed esophagogastrostomy well perfused conduit, no leaks, wound infection or sepsis with very few patients developing anastomotic strictures, requiring intervention were observed (40,41).
Retrosternal bypass with either gastric or colonic conduit had been used in the past for palliation of the dysphagia in cases of locally advanced unresectable esophageal cancer, frequently complicated by tracheoesophageal fistula (26,29,42). However, due to high morbidity and mortality this morbid procedure has all but disappeared from the clinical practice. This process has been facilitated by development of other, less dramatic palliative options—chemotherapy, radiation therapy and endoluminal palliation techniques, such as photodynamic therapy (PDT), brachytherapy, laser and cryoablation and self-expandable stents (43-48). Leaders in the field have condemned surgical esophageal bypass as ill-advised due to high rate of morbidity and mortality (37,49).
Malignant esophageal to airway fistulas leads to uncontrolled airway soilage, pneumonia, severe sepsis and is complicated by high rates of mortality (35). Esophageal bypass has been demonstrated to control soilage and palliate symptoms, again at the cost of significant surgical morbidity and mortality (42).
In pediatric population substernal colon interposition had been used for reconstruction of esophageal atresia with tracheoesophageal fistulas. Long term functional outcomes had been satisfactory with adequate growth of the patient (27,50).
In cases of caustic esophageal injury patients develop severe fibrotic reaction and long recalcitrant strictures. Substernal bypass had been utilized with success in this challenging patient population. Frequently, native esophagus had been left intact due to challenges of dissection in severely fibrotic mediastinum (23,51,52). However, due to increased risk of malignancy many authors propose removal of the diseased esophagus with substernal reconstruction (53).
Minimally invasive, and especially robotic surgery, has been demonstrated to improve outcomes with decreased morbidity, mortality, length of stay and postoperative recovery (2,3,54). For this reason, it has seen rapid adoption into the field of complex esophageal surgery (4,55,56). Minimally invasive and robotic techniques of substernal esophageal reconstruction have been successfully reported in recent literature (55,57,58). With increasing complexity of robotic esophageal procedures routinely performed in the clinical practice, practicing surgeons need to be aware of such advanced reconstructive options as retrosternal bypass and gastric conduit reconstruction.
Authors experience
Authors have performed robotic substernal esophageal bypass and reconstruction in four cases.
A 63-year-old male patient during attempted resection of esophageal adenocarcinoma developed intraoperative bleeding with hemorrhagic shock, complicated by pneumonia and septic shock, forcing surgeon to avoid immediate reconstruction and resort to diversion esophagostomy. After successful recovery patient was brought for retrosternal reconstruction 3 months postoperatively. Procedure was complicated by anastomotic leak, resolving with conservative management and endoluminal VAC therapy within a month with restoration of oral nutrition.
A 71-year-old female patient was transferred from outside facility in shock due to esophageal perforation and leak after minimally invasive repair of paraesophageal hernia. Patient underwent esophageal diversion for control of the source of sepsis. After successful recovery patient underwent reconstruction of gastrointestinal (GI) continuity via substernal gastric conduit reconstruction 4 months after diversion. She has recovered well without leak and full restoration of oral nutrition.
A 42-year-old male, presented in sepsis with pneumonia due to malignant bronchoesophageal fistula due to esophageal squamous cell carcinoma, failed to control with covered esophageal stent. After medical optimization with parenteral nutrition and antibioticotherapy, patient underwent substernal gastric conduit esophageal bypass. After prolonged ICU and hospital course patient has restored oral nutrition and underwent palliative chemotherapy with concurrent radiation therapy with no evidence of disease 2 years after procedure
A 46-year-old male with a history of idiopathic thrombocytopenic purpura (ITP), bilateral above-the-knee amputations (AKA), sepsis, ischemic esophagitis with recalcitrant esophageal stricture developed a bronchoesophageal fistula, failed to control with the stenting, requiring diverting esophagostomy. After medical optimization, 7 months later, the patient underwent robotic retrosternal gastric conduit reconstruction. Postoperative course was complicated by leak, resolving with restoration of oral nutrition 2 months postoperatively.
Technical steps of the substernal reconstruction
Equipment and supplies
We recommend performing esophagectomies on DaVinci Xi surgical platform. It offers several advantages, including beam rotation, allowing docking on either side of the table, camera hoping between ports, wide range of motion, extended length of the instruments, simultaneous use of energy in multiple instruments and lesser spacing between the ports. Whereas surgeon is forced to perform procedure on the Si platform, adjustments to accommodate some of the shortcomings of the older platform are necessary. With progress of the technology and ongoing upgrades of the BaVinci robots in practice, it is expected to see gradual replacement of Si model with time.
Anesthesia and patient setup
Procedure is performed under general endotracheal anesthesia. Abdominal and substernal part of the procedure is performed under singe lumen anesthesia. Most of the time this procedure is performed for reconstruction of previous esophageal diversion. As such, in the majority of cases esophageal resection had been completed previously. When thoracic part is planned for esophageal resection, such as esophageal exclusion in tracheoesophageal fistula, standard lateral position of the patient and docking is required. In these cases, we strongly prefer bronchial blocker for the purpose of lung isolation (Figure 3). Its lower profile and rigidity provide easier and safer dissection of the cervical esophagus. At the end of the case it allows simple conversion to single lumen by removal of the blocker, avoiding potentially dangerous reintubation for toilet bronchoscopy or in case of prolonged ventilation.
Steps of the procedure
Intraoperative endoscopy
After intubation, on-table endoscopy is performed to define the anatomy. In cases of delayed reconstruction after previous cervical esophagostomy, the esophageal stump needs to be assessed for usability and margins. In the cases of planned palliative resection or esophageal bypass, careful assessment of the anatomy is required. Assessment of the stomach is rarely possible as it is either previously disconnected in cases of diversion esophagostomy or might not be accessible due to high grade stricture or malignancy. Review of cross-sectional imaging or previous endoscopy is paramount.
Colonic conduit requires preoperative colonoscopy to assure absence of pathology, precluding its use as a conduit.
Patient positioning
Patient is positioned supine (Figure 4). Shoulder roll is placed for neck hyperextension. Head is rotated to the right for left cervicotomy and esophageal dissection. The right arm is abducted, and the left arm is tucked along the body. Gel roll under the left flank provides slight rotation of the patient facilitating intraabdominal exposure (Figure 5). Wide surgical field prep is applied including neck, chest and abdomen.
Cervicotomy, thoracic inlet enlargement and dissection of the esophagus
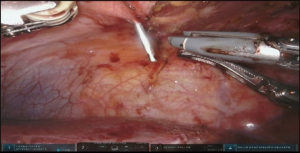
Initial dissection of the neck is started prior to docking of the robot for abdominal part to have a free unobstructed access to the neck. Oblique incision is created along anterior border of left sternocleidomastoid muscle with the extension onto the manubrium (Figure 6 and Figure 7, 00:08). Dissection is carried medial to carotid sheath until prevertebral fascia is encountered and retroesophageal space is exposed. Thyroid laryngeal complex is rotated medially. Attention should be paid to the integrity of the recurrent laryngeal nerve and contact with it should be maximally avoided. Use of self-retaining metal retractors is not recommended for this reason.

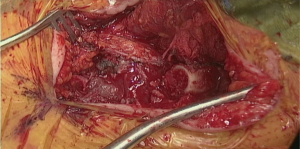
In patients with previous diversion, the esophagostomy is dissected from the skin and the stump is delivered into the cervicotomy wound (Figure 6). In case of bypass, the esophagus is mobilized with blunt dissection from the membranous portion of the trachea. Dissection is carried maximally distally into the posterior mediastinum and esophagus is divided with TA-stapler. If thoracic mobilization was performed during thoracic part for esophageal resection, Penrose drain, placed into the thoracic inlet helps with dissection and delivering the esophageal stump into the wound.
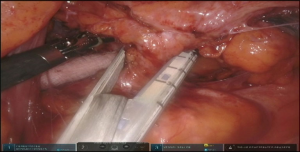
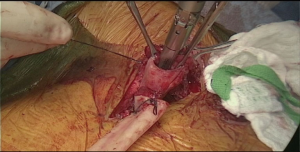
In order to avoid conduit compression with retrosternal route, enlargement of the thoracic inlet aperture is required. Partial left manubriectomy with distal claviculectomy and first rib resection is required for this purpose (Figure 7, 00:24; Figure 8). After opening of the interclavicular ligament, retrosternal space is developed with blunt digital dissection. The heads of the sternocleidomastoid muscle (SCM) are mobilized off the manubrium and distal clavicle. Left pectoralis major muscle is raised off the sternum. Intercostal muscle of the first interspace is mobilized of the manubrium. Attention is paid to avoid left internal mammary pedicle. With the use of the sternal or oscillating saw manubrium is divided at the midline to the lower level of the first intercostal space. Horizontal manubriotomy is performed from the left lateral aspect of the manubrium at the first intercostal space to meet the vertical cut at the midline. Distal clavicle is encircled with right angle clamp and divided with Gigli saw approximately an inch lateral to the SCJ. Underlying first rib is divided at its cartilaginous portion with rib cutter (Figure 9). With blunt digital dissection tunnel is created distally over the pericardium and is packed with surgical gauze for hemostasis, pending transabdominal dissection.
Abdominal port placement
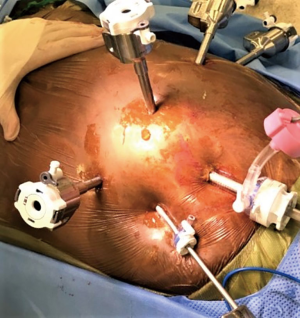
Abdominal part of the procedure is started simultaneously with cervicotomy, pending docking of the robot until completion of the later. Equipment and supplies for the procedure are listed in Table 1. Access to the peritoneal cavity is gained in left periumbilical area with either Veress needle or 5 mm optical trocar. 15 mm of carboxy pneumoperitoneum is created. The robotic camera port is placed in the left paramedian area, 8 cm above the umbilicus. Standard 8 mm robotic ports are placed under the costal margin at the left anterior axillary line, left mid-clavicular and at the right mid clavicular line. In case of use of robotic stapler left arm port is replaced with 12 mm robotic port. The 5-mm port is placed in the right flank and a Mediflex® (Islandia, NY, USA) retractor is used for liver elevation. Nathanson retractor can help localize sternal part of the diaphragm prior to tunnel dissection. Assistant 12 mm laparoscopic post is placed in periumbilical area.
Table 1
| Robotic equipment |
| DaVinci Xi platform |
| Four 8-mm ports |
| 12 mm port (for robotic stapling) |
| 30- and 0-degree cameras |
| Cadiere bipolar grasper |
| Vessel sealer |
| Tip-up grasper |
| Robotic stapler |
| Robotic needle driver |
| Robotic scissors |
| Laparoscopic instruments |
| Laparoscopic tower |
| 5-mm 0- and 30-degree cameras |
| Veress needle or 5 mm optical port |
| 12-mm assistant port |
| Mediflex liver retractor with bed mount |
| Laparoscopic suction irrigator |
| Laparoscopic grasper |
| Laparoscopic scissors |
| Endo Stitch™ device with #2-0 Ethibond sutures |
| Laparoscopic linear stapler |
| Other supplies |
| Gigli saw |
| Sternal or oscillating saw |
| Rib cutter and roungeur |
| Skin stapler |
| 14-Fr jejunostomy tube with introducer kit |
| Silk #1 and # 2-0 |
| Vicryl #2-0 |
| Prolene #2-0 |
| ½ inch Penrose drain |
| TA stapler |
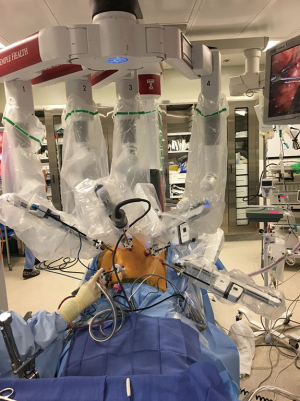
The patient is placed in a reverse Trendelenburg position, and the robot is docked (Figure 10). A 30-degree down camera is used. Bipolar Cadiere grasper is used in arm 1, vessel sealer in arm 3 and a tip up grasper in arm 4.
If enteral access has been established previously it may interfere with port placement. In case of gastrostomy, ports need to be placed below for its dissection and take down. To control the spillage, gastric wall defect can be over sewn or closed with the linear stapler. This defect preferably to be excluded from the conduit during stapling.
Jejunostomy can be preserved for postoperative enteral access and left-flank ports must be placed above and medial to it to avoid interference with range of motion and manipulation.
Gastric dissection and conduit preparation
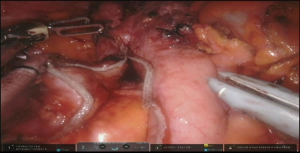
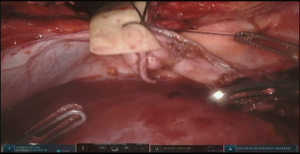
Dissection of the stomach and preparation of the conduit starts with hiatus inspection. In case of previous diversion, gastro esophageal junction (GEJ) is divided and hiatus is closed. Some lysis of adhesions in this area might be required (Figure 7, 00:49). Otherwise the stomach is retracted laterally and pars flaccida of the gastrohepatic ligament is opened with the vessel sealer, exposing the right crus of the diaphragm. The phrenoesophageal membrane is opened circumferentially and high mediastinal dissection and mobilization of the esophagus is performed. Esophagus is divided with the linear stapler as proximally as possible and is delivered into the abdomen. Prior to the esophageal division, nasogastric tube, if present, is pulled back above the level of transection. If thoracic part was performed, the Penrose drain, positioned around distal esophagus helps with the hiatal dissection.
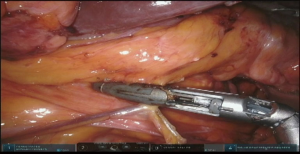
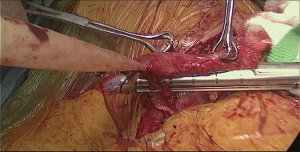
At this point the stomach is retracted medially and cranially and gastrocolic ligament is splayed with lateral traction. With the use of the vessel sealer, the gastrocolic ligament is divided over the mid-body, carefully preserving the integrity of the gastroepiploic pedicle. Complete mobilization of the greater curvature is performed (Figure 11). An omental flap, based on the short gastric vessels in the mid-body, can be harvested for coverage of the gastroesophageal anastomosis.
The stomach is retracted laterally and left gastric pedicle is exposed with dissection of the lymphatic tissue toward the specimen in oncologic cases. Either robotic vascular-load stapler passed through the left subcostal port or standard linear stapler through the assistant port is used for division of the left gastric pedicle (Figure 7, 02:08; Figure 12).
The gastric antrum is retracted medially for the Kocher maneuver and lateral attachments of the duodenum are divided. Adhesions from previous cholecystectomy can present a challenge and need to be mobilized as well. Upon completion of the dissection the pylorus should be easily reaching to the level of hiatus.
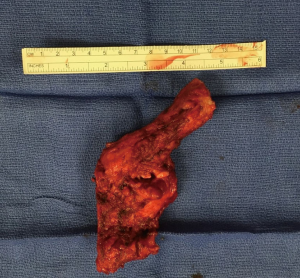
Narrow gastric conduit is created by sequential stapling of the stomach along the greater curvature (Figure 7, 02:53; Figure 13). Stomach is splayed by applying traction laterally and cranially at the fundus and laterally and caudally at the antrum. Starting at the incisura with the linear stapler approximately 5-cm wide gastric tube is fashioned. Attention should be paid to avoid spiraling of the conduit or folding of the posterior gastric wall, by perfect orientation of the retracting arms and the stapler.
Pyloric drainage
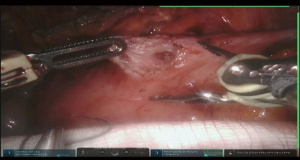
Use of robotic technology allows precise dissection of the duodenal wall layers and performance of a true pyloromyotomy in the majority of cases. Seromuscular 2-0 silk stitch is placed across the pylorus and muscle fibers are divided over the stitch. Remaining muscular fibers are divided until free bulging mucosa is achieved (Figure 14). Pyloromyotomy is closed in transverse Heineke-Mikulicz fashion (Figure 7, 02:30).
Closure of the hiatus
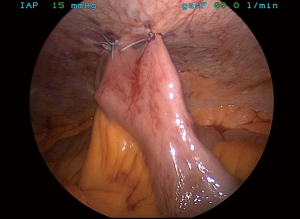
Substernal reconstruction, placing the conduit into the anterior mediastinum calls for complete closure of the hiatus to prevent complications, associated with hernia formation. Hiatus is closed with interrupted #1 silk sutures. Pledges can be used for tissue reinforcement. Usually 4 to 5 sutures are required for complete closure (Figure 15).
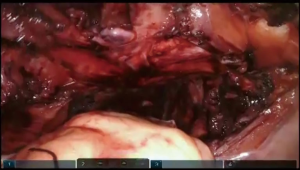
Dissection of the substernal tunnel
The camera is switched to 30-degree up position. Utilizing Veress needle as a finder probe, sternal part of the diaphragm is identified by advancing the needle at the tip of xyphoid process (Figure 7, 03:43; Figure 16). While retracting sternal part of the diaphragm it is opened with the vessel sealer for approximately 5 cm parallel to the sternum. Alternating blunt and sharp dissection the retrosternal tunnel is created over the anterior pericardium, meeting the dissection plane from the neck (Figure 7, 04:24; Figure 17).
Placement of the conduit
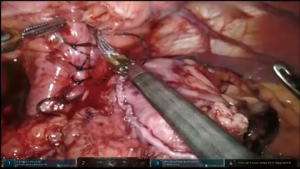
Specimen is extracted through the tunnel after being secured to the Penrose drain, advanced from the cervicotomy (Figure 7, 05:17; Figure 18).
Penrose drain is advanced back through the neck wound into the tunnel, grasped and delivered into the abdomen. Conduit is secured to the drain with two stitches and delivered into the neck incision under visual control from the abdomen to assure proper alignment (Figure 7, 05:41; Figure 19). Additional mobilization of the conduit might be undertaken at this point if required. The conduit is secured to the edge of the diaphragmatic defect to relieve the tension and prevent hernia formation (Figure 20).
Cervical anastomosis
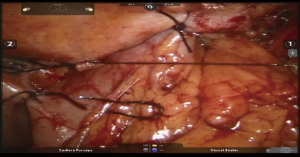
We prefer fully mechanical linear stapler anastomosis in the neck. Conduit is pulled until proper tension to avoid redundancy. Esophageal stump and the tip of the conduit are aligned and the posterior wall stitch is applied. Thick 60 mm linear stapler cartridge is advanced into both lumens and fired, creating anastomosis (Figure 7, 06:16; Figure 21). Naso gastric tube (NGT) is advanced into the distal conduit under direct vision. Anterior aspect of the anastomosis is completed by horizontally firing liner stapler, closing the lumen (Figure 22). Jackson-Pratt drain is placed along the anastomosis and brought out through a separate stab incision. Wound is closed with restoration of the muscular layer between SCM, pectoralis major and contralateral muscles. Skin is closed with staples.
Enteral access
If not previously established, jejunostomy is performed at this point in the preferred fashion. Since placement of the jejunostomy will require redocking of the robot we perform it mainly laparoscopically. Right subcostal, right flank, and assistant ports are used. Ligament of Treitz is identified by elevating colon cranially. Thirty to 50 cm distally loop of small bowel is secured to the abdominal wall in the left flank. With the use an introducer kit a 14 Fr. jejunostomy tube is placed into the lumen. A half−purse-string and an antitorsion stitches are applied (Figure 23).
Postoperative management
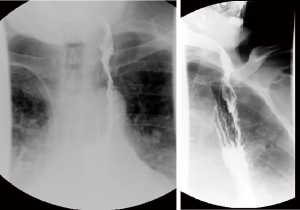
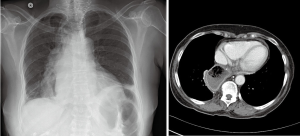
Standard length of hospital stay after robotic esophagectomy is 5 to 7 days. Substernal reconstruction in the open literature was associated with higher rate of morbidity and length of stay. Close monitoring for development of postoperative complications is mandatory. Patient general status, signs of sepsis, quality and quantity of drain output and airleak are inspected frequently. Pressors are avoided intraoperatively and in the postoperative period to avoid compromise of tenuous conduit blood supply. Enteral nutrition is started on postoperative day 2 and advanced as tolerated. Rehabilitation with physical therapy is started on postoperative day (POD) 1. Esophagram with water-soluble contrast and thin barium is performed prior to discharge (Figure 24). Prior to initiation of the oral nutrition speech language pathologist and video barium swallow is recommended to rule out aspirations (Figure 7, 06:45). If anastomotic leak occurs, majority can be managed by opening of the cervical wound for drainage. Endoluminal vacuum assisted closure (VAC) therapy has been shown to hold promise in the management of the leaks. Due to high level of anastomosis and angulation at the level of thoracic inlet we avoid using stent for the management of leaks.
Postoperative surveillance is performed with cross-sectional imaging in cases of malignancy according to the stage of the disease and practice guidelines (Figure 7, 06:59; Figure 25).
Conclusions
In summary, substernal reconstruction is a rare procedure that can be utilized in difficult scenarios for this select group of patients. Robotic technology is feasible, potentially decreasing morbidity of this complex procedure. Surgeons with the interest in complex esophageal procedures should have it in their armamentarium for the best patients’ outcomes.
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA006927.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Robotic Surgery for Esophageal Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.04.02). The series “Robotic Surgery for Esophageal Cancer” was commissioned by the editorial office without any funding or sponsorship. AEA served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Visualized Surgery from Jul 2018 to Jun 2020. RVP reports other (speaker fees) from Veran Medical, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torek F. The first successful case of resection of the thoracic portion of the esophagus for carcinoma. Surg Gynecol Obst 1913;16:614.
- Bakhos CT, Fabian T, Oyasiji TO, et al. Impact of the surgical technique on pulmonary morbidity after esophagectomy. Ann Thorac Surg 2012;93:221-6; discussion 226-7. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: Review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Abbas AE, Dylewski MR. Robotic assisted minimally invasive esophagectomy. In: Kim K. editor. Robotics in general surgery. New York, NY: Springer; 2014.
- Bartels H, Thorban S, Siewert JR. Anterior versus posterior reconstruction after transhiatal oesophagectomy: A randomized controlled trial. Br J Surg 1993;80:1141-4. [Crossref] [PubMed]
- Moremen JR, Ceppa DP, Rieger KM, et al. Substernal reconstruction following esophagectomy: Operation of last resort? J Thorac Dis 2017;9:5040-5. [Crossref] [PubMed]
- Chen H, Lu JJ, Zhou J, et al. Anterior versus posterior routes of reconstruction after esophagectomy: A comparative anatomic study. Ann Thorac Surg 2009;87:400-4. [Crossref] [PubMed]
- Chan ML, Hsieh CC, Wang CW, et al. Reconstruction after esophagectomy for esophageal cancer: Retrosternal or posterior mediastinal route? J Chin Med Assoc 2011;74:505-10. [Crossref] [PubMed]
- Orringer MB. Reversing esophageal discontinuity. Semin Thorac Cardiovasc Surg 2007;19:47-55. [Crossref] [PubMed]
- Gawad KA, Hosch SB, Bumann D, et al. How important is the route of reconstruction after esophagectomy: A prospective randomized study. Am J Gastroenterol 1999;94:1490-6. [Crossref] [PubMed]
- Zheng YZ, Dai SQ, Li W, et al. Comparison between different reconstruction routes in esophageal squamous cell carcinoma. World J Gastroenterol 2012;18:5616-21. [Crossref] [PubMed]
- Urschel JD. Does the interponat affect outcome after esophagectomy for cancer? Dis Esophagus 2001;14:124-30. [Crossref] [PubMed]
- van Lanschot JJ, van Blankenstein M, Oei HY, et al. Randomized comparison of prevertebral and retrosternal gastric tube reconstruction after resection of oesophageal carcinoma. Br J Surg 1999;86:102-8. [Crossref] [PubMed]
- Coral RP, Constant-Neto M, Silva IS, et al. Comparative anatomical study of the anterior and posterior mediastinum as access routes after esophagectomy. Dis Esophagus 2003;16:236-8. [Crossref] [PubMed]
- Koskas F, Gayet B. Anatomical study of retrosternal gastric esophagoplasties. Anat Clin 1985;7:237-56. [Crossref] [PubMed]
- Hu H, Ye T, Tan D, et al. Is anterior mediastinum route a shorter choice for esophageal reconstruction? A comparative anatomic study. Eur J Cardiothorac Surg 2011;40:1466-9. [PubMed]
- Yang J, Xu C, Lian D, et al. Esophageal reconstruction: Posterior mediastinal or retrosternal route. J Surg Res 2016;201:364-9. [Crossref] [PubMed]
- Wang H, Tan L, Feng M, et al. Comparison of the short-term health-related quality of life in patients with esophageal cancer with different routes of gastric tube reconstruction after minimally invasive esophagectomy. Qual Life Res 2011;20:179-89. [Crossref] [PubMed]
- Tobinaga S, Tayama K, Kawano H, et al. Aortic valve replacement after esophagectomy with substernal gastric tube reconstruction. Ann Thorac Cardiovasc Surg 2006;12:213-5. [PubMed]
- Iemura J, Oku H, Ohtaki M, Inoue T. Coronary artery bypass grafting following substernal gastric interposition. Jpn Circ J 2000;64:404-5. [Crossref] [PubMed]
- Kashiyama N, Toda K, Miyagawa S, et al. Off-pump coronary artery bypass grafting via median sternotomy in a patient with a history of esophagectomy with substernal gastric tube reconstruction: Report of a case. Surg Today 2015;45:1190-3. [Crossref] [PubMed]
- Pavliuk AD. Substernal reconstruction of subcutaneous artificial esophagus and removal of cutaneous patch. Klin Khir 1994;67-8. [PubMed]
- Ein SH. Gastric tubes in children with caustic esophageal injury: A 32-year review. J Pediatr Surg 1998;33:1363-5. [Crossref] [PubMed]
- Sugimachi K, Kitamura M, Maekawa S, et al. Two-stage operation for poor-risk patients with carcinoma of the esophagus. J Surg Oncol 1987;36:105-9. [Crossref] [PubMed]
- Chung JH, Lee SH, Yi E, et al. A non-randomized retrospective observational study on the subcutaneous esophageal reconstruction after esophagectomy: Is it feasible in high-risk patients? J Thorac Dis 2017;9:675-84. [Crossref] [PubMed]
- Orringer MB, Sloan H. Substernal gastric bypass of the excluded thoracic esophagus for palliation of esophageal carcinoma. J Thorac Cardiovasc Surg 1975;70:836-51. [PubMed]
- Appignani A, Lauro V, Prestipino M, et al. Intestinal bypass of the oesophagus: 117 patients in 28 years. Pediatr Surg Int 2000;16:326-8. [Crossref] [PubMed]
- Meunier B, Stasik C, Raoul JL, et al. Gastric bypass for malignant esophagotracheal fistula: A series of 21 cases. Eur J Cardiothorac Surg 1998;13:184-8; discussion 188-9. [Crossref] [PubMed]
- Meunier B, Spiliopoulos Y, Stasik C, et al. Retrosternal bypass operation for unresectable squamous cell cancer of the esophagus. Ann Thorac Surg 1996;62:373-7. [Crossref] [PubMed]
- Conlan AA, Nicolaou N, Hammond CA, et al. Retrosternal gastric bypass for inoperable esophageal cancer: A report of 71 patients. Ann Thorac Surg 1983;36:396-401. [Crossref] [PubMed]
- Urschel JD, Urschel DM, Miller JD, et al. A meta-analysis of randomized controlled trials of route of reconstruction after esophagectomy for cancer. Am J Surg 2001;182:470-5. [Crossref] [PubMed]
- Barkley C, Orringer MB, Iannettoni MD, et al. Challenges in reversing esophageal discontinuity operations. Ann Thorac Surg 2003;76:989-94; discussion 995. [Crossref] [PubMed]
- DiPierro FV, Rice TW, DeCamp MM, et al. Esophagectomy and staged reconstruction. Eur J Cardiothorac Surg 2000;17:702-9. [Crossref] [PubMed]
- Dickinson KJ, Blackmon SH. Management of conduit necrosis following esophagectomy. Thorac Surg Clin 2015;25:461-70. [Crossref] [PubMed]
- Rigberg DA, Centeno JM, Blinman TA, et al. Two decades of cervical esophagostomy: Indications and outcomes. Am Surg 1998;64:939-941. [PubMed]
- Lee YC, Lee ST, Chu SH. New technique of esophageal exclusion for chronic esophageal perforation. Ann Thorac Surg 1991;51:1020-2. [Crossref] [PubMed]
- Orringer MB. Substernal gastric bypass of the excluded esophagus--results of an ill-advised operation. Surgery 1984;96:467-70. [PubMed]
- Moore JM, Hooker CM, Molena D, et al. Complex esophageal reconstruction procedures have acceptable outcomes compared with routine esophagectomy. Ann Thorac Surg 2016;102:215-22. [Crossref] [PubMed]
- Oida T, Mimatsu K, Kano H, et al. Anterior vs. posterior mediastinal routes in colon interposition after esophagectomy. Hepatogastroenterology 2012;59:1832-4. [PubMed]
- Oezcelik A, Banki F, DeMeester SR, et al. Delayed esophagogastrostomy: A safe strategy for management of patients with ischemic gastric conduit at time of esophagectomy. J Am Coll Surg 2009;208:1030-4. [Crossref] [PubMed]
- Lanzarini E, Ramon JM, Grande L, et al. Delayed cervical esophagogastrostomy: A surgical alternative for patients with ischemia of the gastric conduit at time of esophagectomy. Cir Esp 2014;92:429-31. [Crossref] [PubMed]
- Campion JP, Bourdelat D, Launois B. Surgical treatment of malignant esophagotracheal fistulas. Am J Surg 1983;146:641-6. [Crossref] [PubMed]
- May A, Ell C. Palliative treatment of malignant esophagorespiratory fistulas with gianturco-Z stents. A prospective clinical trial and review of the literature on covered metal stents. Am J Gastroenterol 1998;93:532-5. [PubMed]
- Martin RC 2nd, Cannon RM, Brown RE, et al. Evaluation of quality of life following placement of self-expanding plastic stents as a bridge to surgery in patients receiving neoadjuvant therapy for esophageal cancer. Oncologist 2014;19:259-65. [Crossref] [PubMed]
- Włodarczyk JR, Kuzdzal J. Stenting in palliation of unresectable esophageal cancer. World J Surg 2018;42:3988-96. [Crossref] [PubMed]
- Penniment MG, De Ieso PB, Harvey JA, et al. Palliative chemoradiotherapy versus radiotherapy alone for dysphagia in advanced oesophageal cancer: A multicentre randomised controlled trial (TROG 03.01). Lancet Gastroenterol Hepatol 2018;3:114-24. [Crossref] [PubMed]
- Sreedharan A, Harris K, Crellin A, et al. Interventions for dysphagia in oesophageal cancer. Cochrane Database Syst Rev 2009;CD005048 [PubMed]
- Sharma V, Mahantshetty U, Dinshaw KA, et al. Palliation of advanced/recurrent esophageal carcinoma with high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2002;52:310-5. [Crossref] [PubMed]
- Alcantara PS, Spencer-Netto FA, Silva-Junior JF, et al. Gastro-esophageal isoperistaltic bypass in the palliation of irresectable thoracic esophageal cancer. Int Surg 1997;82:249-53. [PubMed]
- Henry CL, Reinerssman JM, Deb SJ. Substernal colon volvulus with ischemia 43 years after reconstruction for esophageal atresia. Am Surg 2017;83:e396-7. [PubMed]
- Raffensperger JG, Luck SR, Reynolds M, et al. Intestinal bypass of the esophagus. J Pediatr Surg 1996;31:38-46; discussion 46-7. [Crossref] [PubMed]
- Ramareddy RS, Alladi A. Review of esophageal injuries and stenosis: Lessons learn and current concepts of management. J Indian Assoc Pediatr Surg 2016;21:139-43. [Crossref] [PubMed]
- Rodgers BM, Ryckman FC, Talbert JL. Blunt transmediastinal total esophagectomy with simultaneous substernal colon interposition for esophageal caustic stricture in children. J Pediatr Surg 1981;16:184-9. [Crossref] [PubMed]
- Palazzo F, Rosato EL, Chaudhary A, et al. Minimally invasive esophagectomy provides significant survival advantage compared with open or hybrid esophagectomy for patients with cancers of the esophagus and gastroesophageal junction. J Am Coll Surg 2015;220:672-9. [Crossref] [PubMed]
- Petrov R, Bakhos C, Abbas A. Robotic-assisted minimally invasive esophagectomy. In: Kudsi Y, Carbonell A, Yiengpruksawan A, et al. editors. Atlas of robotic surgery. Woodbury, CT: Cine-Med.; 2018.
- Cerfolio RJ, Wei B, Hawn MT, et al. Robotic esophagectomy for cancer: Early results and lessons learned. Semin Thorac Cardiovasc Surg 2016;28:160-9. [Crossref] [PubMed]
- Mungo B, Barbetta A, Lidor AO, et al. Laparoscopic retrosternal gastric pull-up for fistulized mediastinal mass. World J Gastrointest Surg 2017;9:92-96. [Crossref] [PubMed]
- Petrov R, Bakhos C, Abbas A. Robotic esophagectomy. In: Koy TS. editor. Robotic-assisted minimally invasive surgery. Switzerland: Springer Nature.; 2019:277-93.
- Petrov RV, Bakhos CT, Abbas AE. Substernal gastric conduit reconstruction. Asvide 2019;6:127. Available online: http://www.asvide.com/article/view/31470
Cite this article as: Petrov RV, Bakhos CT, Abbas AE. Robotic substernal esophageal bypass and reconstruction with gastric conduit—frequently overlooked minimally invasive option. J Vis Surg 2019;5:47.