
Transhiatal robot assisted minimally invasive esophagectomy (Th-RAMIE)
Esophageal cancer is estimated to be found in over 17,000 people in the US in 2019 with over 16,000 deaths (1). Resection, either alone by endoluminal resection or esophagectomy for early stage disease or esophagectomy combined with chemotherapy and/or radiotherapy, offers the best chance for long-term survival (2). There are three traditional approaches that have been described for esophagectomy: transthoracic (Ivor-Lewis esophagectomy) with a mediastinal anastomosis, transhiatal with a cervical anastomosis, and the three-hole (McKeown) esophagectomy. As minimally invasive approaches have been adopted, they have not fundamentally changed the operations, but have converted the open laparotomy and/or thoracotomy to laparoscopy and/or thoracoscopy, and are all modifications of the transhiatal, transthoracic, or three-field operations. Robotics have introduced an alternative option to traditional minimally invasive techniques for abdominal and thoracic work. This review will focus on the operative technique of a robotic-assisted transhiatal approach, including intra- and postoperative complications compared to the open transhiatal approach (3) (Figure 1).

Preoperative evaluation
Careful patient selection and preoperative evaluation are essential to the success of any esophagectomy. For patients undergoing esophagectomy, preoperative preparation, including smoking cessation and ambulation, is as important in decreasing postoperative morbidity and mortality as the technical aspects of the operation itself. For patients with malnutrition or severe dysphagia, a feeding nasogastric or laparoscopic jejunostomy tube may be placed and tube feeds initiated as needed prior to the planned esophagectomy.
Operative technique
Following induction of general anesthesia using a single-lumen endotracheal tube, a flexible esophagogastroduodenoscopy is performed with special attention paid to the location and extent of the esophageal tumor, the gastric extension of tumor as seen on retroflexion, and the status of the pylorus. After removal of the endoscope, a nasogastric tube is placed to decompress the stomach and to aid in mobilizing the esophagus in the neck.
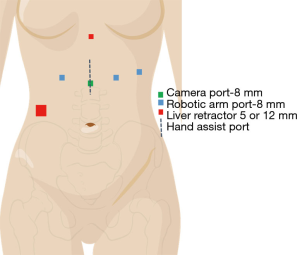
Patient positioning and port placement (Xi platform)
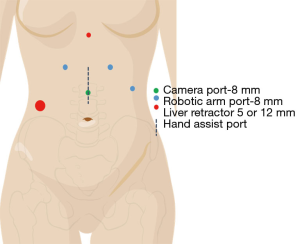
The patient is positioned supine with a rolled blanket placed behind the shoulders to facilitate neck extension with the head is turned to the right and supported while the arms are tucked at the sides. The neck, chest and abdomen should be prepped into the operative field. Ports are placed (Figure 2, Xi port placement) for the laparoscopic dissection, beginning by marking the camera port 11 cm below the xiphoid process. Two port sites are marked at 6 and 12 cm to the patient’s left from the camera port (Right arms 1 and 2) with the third arm port placed 6 cm to the patient’s right from the camera port. A 12 mm port is placed in the right lower quadrant through which a paddle liver retractor is placed. An alternative liver retractor is the Nathanson, which is placed through a 5 mm port just below the Xiphoid process. The Nathanson can work well, but occasionally can cause liver trauma and bleeding through the operation. The paddle retractor minimizes this risk. The camera port incision is made vertically, while the other incisions are made in a transverse fashion. The camera is placed 30 degrees down in the midline port site, with a Prograsp placed in the Left arm, and a Vessel Sealer through Right arm 1, and a Tip-Up through Right arm 2. Alternative port site placement for the Si platform is outlined in Figure 3 with similar instrument use.
Short gastric artery and left crus mobilization
The upper abdomen is explored for metastatic disease. The empty space between the gastroepiploic and the short gastric artery arcade is identified. The tip up is used to retract. The short gastric arteries should be ligated by the Vessel sealer towards the left crus. The phrenoesophageal ligament is taken down from roughly 5 to 12 o’clock, with dissection carried out 2–3 cm beyond the crus into the chest.
Right crus mobilization and division of the left gastric vessels
The gastrohepatic ligament is examined for an aberrant left hepatic artery originating from the left gastric artery. If an aberrant hepatic artery is identified, it should be preserved and the left gastric artery divided distal to the origin of the aberrant artery with the Vessel sealer. Once the ligament is divided, the right crus is exposed and then mobilized from 7 to 12 o’clock to again 2–3 cm above the crus. If there is no aberrant artery, the fat in the left gastric artery pedicle is divided near its origin from the celiac trunk ensuring adequate lymph node procurement. The left gastric artery and vein are then dissected and divided with either a vessel sealer or by stapler through an assistant port or an upsized 12 mm robotic port. Once the left gastric artery is taken, the area of the crus from 5 to 7 o’clock is mobilized again above the diaphragm.
Mediastinal mobilization
Using the Tip-up to retract the gastroesophageal junction, one can continue with mediastinal mobilization. Care should be taken to avoid getting into the pleural spaces if possible as once they are entered, the mediastinal space can collapse and one then loses exposure. As one mobilizes higher than 3–4 cm above the diaphragm, the camera view should be switched to a 30 degree up view, which allows a view “up” the mediastinum. This facilitates the exposure of the carinal space to allow the resection of the subcarinal lymph nodes and to better view the aorta, carina, and the azygous vein. Small chest tubes or drains can be placed prior to robotic dissection bilaterally, or if pleural injuries are noted. It can be challenging but not impossible to place a chest tube around a docked robot.
Robotic Kocher maneuver (Xi only)
With the Xi, one can rotate the camera and instruments to look down at the duodenum. The Tip-up can be used to pull the duodenum to the patient’s left, and then the vessel sealer can open up the tissue plane to expose the inferior vena cava (IVC). A generous Kocher maneuver is completed until the pylorus is able to reach the level of the hiatus. If an Si is being used, the Kocher can be deferred to after the hand-assist port is placed. Laparotomy pads and sweetheart retractors can be used to push the transverse colon inferiorly and the duodenum and stomach to the patient’s left to gain exposure. A long right angle can be used to expose the plane between the duodenum and IVC.
Hand port, completion of gastric mobilization, pyloromyotomy, J-tube placement
The robotic ports are removed, but the liver retractor is kept in place. A 7 cm midline is incision is made, roughly 1–2 cm inferior to the camera port, and 5 cm above it. A gel port is placed to retract the abdominal wall. The stomach is further mobilized, taking care to preserve the gastroepiploic artery by dividing the omental branches off of the gastroepiploic from the level of the pylorus, along the greater curve to the take off of the short gastric arteries. Posterior gastric adhesions to the pancreas are also lysed at this time. A 2-cm long pyloromyotomy is performed. If the mucosa is entered, the injury should be repaired with small absorbable monofilament suture and covered with a Graham patch or converted to a pyloroplasty. A 14-Fr rubber jejunostomy feeding tube is placed 20 cm beyond the ligament of Treitz and secured in place using a Witzel maneuver. The tube is temporarily clamped using three large clips and then tucked into the abdomen with the gel cover placed on the port.
Mobilization of the cervical esophagus
A 7 cm cervical incision is made parallel to the anterior border of the left sternocleidomastoid (SCM) muscle. The omohyoid muscle is identified and divided. Care is taken to avoid direct pressure on the recurrent laryngeal nerve in the tracheoesophageal groove. The carotid sheath is entered, with the inferior thyroid artery and middle thyroid vein are divided for better exposure. The prevertebral fascia is identified and blunt finger dissection is used to define the esophagus. The space between the trachea and esophagus is divided using sharp dissection. A finger is placed over the esophagus pulling the nasogastric tube and esophagus to the left. A Penrose drain is placed around the cervical esophagus and retraction facilitates the blunt mobilization of the upper thoracic esophagus from the superior mediastinum.
Mobilization of the thoracic esophagus
Mobilization of the thoracic esophagus is completed by removing the gel cover and placing a hand at the esophageal hiatus with at least two fingers placed through the hiatus under the esophagus. Working downward through the cervical incision using a curved sponge stick, dissection is first carried out along the posterior aspect of the esophagus staying as close to the spine as possible to avoid compressing the heart. Once the posterior dissection is completed, a 28-Fr Argyle Saratoga sump catheter is inserted through the cervical incision to evacuate blood from the mediastinum. Dissection of the esophagus then proceeds along the lateral attachments using a standard sucker tip placed through the neck to reach down to the fingers through the hiatus. Lastly, the anterior attachments are taken down by dissecting posteriorly toward the esophagus and away from the posterior pericardium and membranous trachea. If the mediastinal dissection has been carried to 2 cm above the carina, there is often no need for further blind mediastinal dissection.
Division of the cervical esophagus and creation of the gastric conduit
Once the entire intrathoracic esophagus has been mobilized from the mediastinum, the upper esophagus is delivered into the cervical wound and the nasogastric tube is pulled back into the oropharynx. The esophagus is divided with a gastrointestinal anastomosis (GIA) surgical stapler in an anterior to posterior direction, leaving more length on the anterior side. An Allis clamp is placed on the staple line of the cervical esophagus to prevent it from retracting into the wound. The stomach and thoracic esophagus are then delivered out of the hand port and a GIA stapler is used to create the conduit, starting 5 cm along the lesser curve, and following the greater curve to allow for more length in the conduit. The gastric staple line is oversewn with a running 4-0 Prolene Lembert stitch. Our practice is to then assess the gastric conduit for perfusion by laying the conduit flat on a green towel over the abdomen using near infrared spectroscopy (SPY©). Indocyanine green dye is given (5 mg) followed by a 10 mL saline flush and the perfusion is recorded for 120 seconds looking for the speed of perfusion to the conduit proximally and the quality of perfusion to the gastric tip. The gastric tip can be resected in the neck once it is advanced to ensure adequate length. If more length is needed, further mobilization in the abdomen can be performed through the hand port if needed.
Passage of the gastric conduit through the posterior mediastinum
The gastric conduit is gently manipulated through the esophageal hiatus and advanced manually upward through the posterior mediastinum. The fundus is grasped by placing a Babcock clamp through the neck into the mediastinum and gently guided upward while the hand in the hiatus pushes the conduit upwards. A small mosquito clamp is placed on fat near the tip of the gastric conduit to prevent it from retracting back into the mediastinum. The cervical wound is then covered with a moist thoracic pack while the abdominal portion of the case is completed.
Closure of the esophageal hiatus
The three non-midline robotic ports are replaced and the gel cover placed over the hand port with insufflation restarted. The robot is redocked and the camera placed in the middle robotic port with a Pro-grasp in the right abdominal port and a needle driver in the outer left abdominal port. The esophageal hiatus is narrowed to 2 fingerbreadths using interrupted 1-0 silk sutures placed on the top right corner of the crus from 3 to 12 o’clock. A hand is placed through the gel port to confirm adequate narrowing. The ports and retractors are removed. The jejunostomy tube is brought out through the first left upper quadrant port site and tacked to the adjacent peritoneum with interrupted 3-0 silk sutures. The tube is also secured at the level of the skin with a 2-0 Prolene suture. The fascia is then closed in standard fashion and the skin closed with monocryl.
Creation of a stapled cervical esophagogastric anastomosis
The tip of the gastric conduit is pulled out of the neck and a 3-0 silk seromuscular traction suture is placed along the anterior wall as distal as possible. A 1 cm vertical gastrostomy is made in the anterior wall/greater curve of the stomach. A 4-0 Vicryl suture is placed on the distal side of the gastrostomy leaving the needle on the suture. The stapled end of the cervical esophagus is amputated and submitted as the proximal esophageal margin. A second 4-0 Vicryl stay suture is placed on the anterior edge of the esophagus with the first Vicryl used to facilitate alignment of the posterior wall of the esophagus and the anterior wall of the stomach. A side-to-side anastomosis between the cervical esophagus and gastric fundus is performed using the Endo GIA 30-tissue staple load. Once the stapler is closed, two 4-0 Vicryl seromuscular sutures are placed between the esophagus and stomach on both sides of the stapler. The stapler is then fired creating a 3 cm side-to-side anastomosis. The nasogastric tube in the oropharynx is carefully guided across the anastomosis and secured at 40–45 cm from the nares. The anterior opening of the anastomosis is then closed in 2 layers. The inner mucosal layer is closed with running 4-0 PDS, while the outer later is performed with interrupted 4-0 PDS sutures. Clips are used to mark the anastomosis for future radiographic localization.
Closure
The cervical wound is then irrigated and a 1/4-inch Penrose drain is positioned next to the anastomosis. The muscle fascia is loosely approximated with interrupted 3-0 Vicryl and the skin edges are closed with running 4-0 nylon. Dry sterile dressings are applied to all incisions. A postoperative chest radiograph is obtained in the operating room while the patient is intubated to determine the proper placement of chest tubes.
Postoperative management
Our practice is to place an epidural catheter or a rectus sheath block prior to the operation for postoperative analgesia. Patients are extubated in the operating room and admitted to the general thoracic surgery ward. Ambulation is started on postoperative day (POD) 1. The nasogastric tube is initially placed to suction and then to dependent drainage on POD2 and removed on POD3. Trickle tube feeds through the jejunostomy tube are initiated on POD2 and advanced as tolerated. Oral liquids are started on POD4 following removal of the nasogastric tube. The diet is gradually advanced to a soft diet by POD7. Tube feeds are tapered as oral intake improves. Chest tubes are removed when drainage is less than 60 mL per shift for 2 consecutive shifts and if the drainage is serous. The Penrose drain in the neck is removed on POD4 if there are no clinical signs of a leak. A routine barium swallow is obtained on POD7 to document integrity of the anastomosis and adequacy of gastric emptying. Patients are typically discharged from the hospital on the seventh after surgery.
Robotic specific intraoperative complications
Traditional complications, such as azygous vein injury, conduit injury, recurrent laryngeal nerve injury, associated with open transhiatal esophagectomies can occur with the robotic approach as well. Some subtle robotic specific complications that have been noted include delayed recognition of pleural entry resulting in peak airway pressures and tension pneumothorax physiology. Delayed pleural effusions or pneumothoraces can be seen even 48–72 hours post-operatively and thus our practice as shifted to placing early bilateral drains for almost all operations. Thin patients can have subcutaneous emphysema develop during the abdominal dissection which usually resolves by the end of the operation. Early post-operative hiatal hernias of colon or small intestine were noted when the hiatal closure was not done, or performed laparoscopically. Redocking the robot, using 1-0 silk sutures, and using a hand to assess for hiatal narrowing has eliminated this. Attempts to use minimally invasive jejunostomy tubes led to intermitted clogging and rare enterocutaneous fistulas after removing the tubes. This is led us to continue to favor placing Witzeled J-tubes by open technique.
Discussion
There are many publications showing improved outcomes (shorter length of stay, reduced morbidity) after minimally invasive esophagectomies over open esophagectomies (5,6). These studies have shown benefits in esophagectomies that have include thoracotomy approaches. For transhiatal esophagectomies specifically, the data on the benefits of a minimally invasive and/or robotic minimally invasive esophagectomy are less clear. The robotic platform is felt to provide a better lymph node and mediastinal dissection (7,8). To date, there are only small case series ranging from 1–100 Th-RAMIEs showing a proof of concept comparing Th-RAMIE outcomes to historical data (9-13). These series have shown post-operative pleural effusions ranging from 38% (9) to 45% (13) (same authors, and likely overlapping data set), which is consistent with our institutional experience which has shown delayed pulmonary complications at a much higher rate over open transhiatal esophagectomies (unpublished data). We have seen an increase in the number of resected lymph nodes in the Th-RAMIE group over the open ThE group, but have not seen shorter length of stays or other reduction in complications (unpublished data). The transhiatal robotic assisted minimally invasive esophagectomy is clearly feasible and likely offers advantages is short term morbidity over other esophagectomy approaches in a similar fashion that transhiatal does over transthoracic approaches, and may allow for improved lymph node dissection, which has been a concern for surgeons with the transhiatal approach.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Abbas Abbas) for the series “Robotic Surgery for Esophageal Cancer” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.05.07). The series “Robotic Surgery for Esophageal Cancer” was commissioned by the editorial office without any funding or sponsorship. RMR reports personal fees (Teaching Site, Speaker) from Intuitive Surgical, personal fees (Advisory Board Member) from Medtronic, personal fees (Consultant) from Auris Health, outside the submitted work. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Cancer Facts and Figures 2019. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed March 1, 2019.
- PDQ Adult Treatment Editorial Board. Esophageal Cancer Treatment (PDQ®): Patient Version. Available online: https://www.cancer.gov/types/esophageal/patient/esophageal-treatment-pdq
- Orringer MB. Transhiatal esophagectomy without throacotmy. In: Shields TW, Locicero J, Reed CE, et al. editors. General Thoracic Surgery. 7th edition. Philadelphia, PA: LWW, 2009:1771-94.
- Reddy RM. The steps of a Transhiatal Robot (Xi-Davinci) assisted minimally invasive esophagectomy (Th-RAMIE). Asvide 2019;6:177. Available online: http://www.asvide.com/article/view/32356
- Peng JS, Kukar M, Mann GN, et al. Minimally Invasive Esophageal Cancer Surgery. Surg Oncol Clin N Am 2019;28:177-200. [Crossref] [PubMed]
- Murthy RA, Clarke NS, Kernstine KH Sr. Minimally Invasive and Robotic Esophagectomy: A Review. Innovations (Phila) 2018;13:391-403. [Crossref] [PubMed]
- Seto Y, Mori K, Aikou S. Robotic surgery for esophageal cancer: Merits and demerits. Ann Gastroenterol Surg 2017;1:193-8. [Crossref] [PubMed]
- Mori K, Yamagata Y, Wada I, et al. Robotic-assisted totally transhiatal lymphadenectomy in the middle mediastinum for esophageal cancer. J Robot Surg 2013;7:385-7. [Crossref] [PubMed]
- Dunn DH, Johnson EM, Anderson CA, et al. Operative and survival outcomes in a series of 100 consecutive cases of robot-assisted transhiatal esophagectomies. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]
- Coker AM, Barajas-Gamboa JS, Cheverie J, et al. Outcomes of robotic-assisted transhiatal esophagectomy for esophageal cancer after neoadjuvant chemoradiation. J Laparoendosc Adv Surg Tech A 2014;24:89-94. [Crossref] [PubMed]
- Galvani CA, Gorodner MV, Moser F, et al. Robotically assisted laparoscopic transhiatal esophagectomy. Surg Endosc 2008;22:188-95. [Crossref] [PubMed]
- Gutt CN, Bintintan VV, Köninger J, et al. Robotic-assisted transhiatal esophagectomy. Langenbecks Arch Surg 2006;391:428-34. [Crossref] [PubMed]
- Dunn DH, Johnson EM, Morphew JA, et al. Robot-assisted transhiatal esophagectomy: a 3-year single-center experience. Dis Esophagus 2013;26:159-66. [Crossref] [PubMed]
Cite this article as: Reddy RM. Transhiatal robot assisted minimally invasive esophagectomy (Th-RAMIE). J Vis Surg 2019;5:57.


