Robot-assisted minimally invasive mitral valve surgery
Introduction
During robot-assisted mitral valve (RAMV) operations, surgeons translate their hand motions through a console to activate instrument end-effectors that have seven degrees of ergonomic freedom. Moreover, a high definition camera provides magnified 3D vision. Our group performed the first daVinci™ Surgical System robotic mitral repair in the United States in May of 2000 (Intuitive Surgical Inc., Sunnyvale, CA, USA). Since then large clinical series from dedicated referral programs have shown outcomes that are similar to operations done either through a sternotomy or a minimally invasive incision. Not only have RAMV repairs been shown to be durable, but patient safety has been demonstrated with few complications. The benefits have included less bleeding and transfusions, reduced intensive care and hospital stays, and faster recovery than traditional sternotomy operations. The Atlas of Robotic Cardiac Surgery describes in detail the planning and performance of RAMV operations (1).
Indications and contraindications
Patients should have the indications for mitral valve repair surgery as outlined in either the 2014/2017 AHA/ACC (US) or 2017 ESC/EACTS (European) Guidelines (2,3). Combined operations (mitral and tricuspid valve as well as the cryomaze procedure) also can be performed safely using robot-assistance. General and relative contraindications to the robot-assisted approach are listed in Table 1.
Table 1
| Previous right thoracotomy with a lung resection or pleurodesis |
| Poor pulmonary function |
| Pulmonary hypertension (PAS >70 torr)* |
| Pulmonary hypertension with a dilated poorly contracting right ventricle |
| Severe liver dysfunction |
| Bleeding disorders* |
| Significant aortic valve disease |
| Coronary artery disease requiring revascularization* |
| Recent myocardial ischemia (<30 days) |
| Recent stroke (<30 days) |
| Severely calcified mitral valve annulus |
| Severe aorto-iliac atherosclerosis or aneurysm* |
| Severe left ventricular failure (EF <25%) |
*, relative contraindications: some may be managed either by a detailed preoperative evaluation or intervention and/or the use of alternate techniques. PAS, pulmonary artery systolic pressure; EF, ejection fraction.
Pre-operative planning
Either an excellent trans-thoracic or trans-esophageal echocardiographic (TEE) study should be done for diagnosis and to help guide the operative plan. Computed tomographic (CT) images should be obtained in selected patients to define the status of the peripheral vasculature and aorta. Patients at risk for coronary artery disease should have coronary angiography or a CT angiogram. After operating room anesthesia induction, a comprehensive 2D and 3D TEE study should be done to provide the planning information listed in Table 2.
Table 2
| A2 and P1–P3 leaflet segment lengths |
| Mitral leaflet prolapse or restricted regions |
| Direction and numbers of regurgitant jets |
| Posterior leaflet segment indentations |
| Annular posterior-anterior diameter and shape |
| Aorto-mitral valve annular plane angle (<120 degrees) |
| Inter-ventricular septal thickness |
| Coaptation-septal distance |
| Right and left ventricular function |
| Left atrial diameter |
| A topographic valve reconstruction from 3D images |
RAMV surgery
Patient positioning
The patient should be positioned with the right chest elevated by 30°. The right arm should be suspended safely below the posterior axillary line. The right axillary area should be exposed widely for insertion of the trans-thoracic aortic cross clamp. Standard skin preparation and draping must provide wide exposure to the right chest, sternum, and both groins.
Anesthesia management
Our anesthesia preparation includes placing the following (Figure 1):
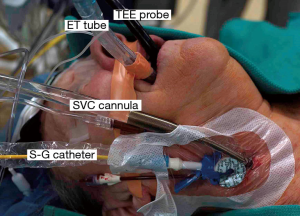
- Either a double-lumen endotracheal tube or a right endo-bronchial blocker;
- A right radial arterial blood pressure monitoring catheter;
- Posterior-anteriorly placed Zoll defibrillator pads;
- A right internal jugular introducer for drug infusions and Swan-Ganz pulmonary artery (PA) catheter insertion;
- A thin-walled (15 or 17 Fr) Bio-Medicus™ (Medtronic, Inc., MN, USA) right internal jugular venous drainage cannula (double-puncture method);
- A 3D TEE probe.
Working incision
A small working port incision (2–3 cm) is made in 4th intercostal space in the anterior axillary line. We place a flexible Alexis™ soft tissue wound protector (Applied Medical, Inc., Rancho Santa Margarita, CA, USA) rather than using a rib-spreading retractor.
Cardiopulmonary perfusion
Using the Seldinger guide-wire technique, all perfusion cannulas are placed under TEE guidance. Through a 2 cm oblique groin incision, the right femoral artery is cannulated with 17 to 21 Fr Bio-Medicus™ cannula (Medtronic, St. Paul, MN, USA). For inferior vena caval drainage either a 22 Fr (single stage) or a 23/25 Fr (dual stage) RAP™ femoral venous cannula (VC; Livanova, London, UK) is passed into the right (Rt.) atrium (Figure 2). Vacuum-assisted venous drainage is used for these operations. Intra-thoracic carbon dioxide insufflation minimizes intra-cardiac air entrapment. During cardiopulmonary bypass (CPB), we monitor leg oxygen saturation levels using the Invos™ System (Somanetics Inc., Troy, MI, USA). If a significant arterial saturation decrease occurs in the cannulated leg, a 5 Fr arterial perfusion circuit catheter is placed in distal superficial femoral artery. Detailed perfusion methods for RAMV procedures are detailed in Cardiopulmonary Bypass and Mechanical Support: Principles and Practice (4).
Aortic occlusion
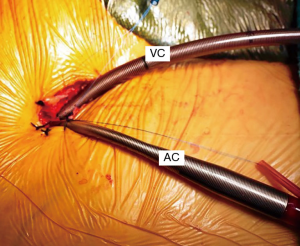
A trans-thoracic cross clamp (TT-XCL; Scanlan International, St. Paul, MN, USA) is used for aortic occlusion and has been proven to be safe, reliable, and simple to apply (Figure 3). The posterior clamp tine should be passed through the transverse sinus under either direct or videoscopic vision. Care must be taken not to injure the right pulmonary artery (RPA), left atrial appendage, left main coronary artery, or aorta (Figure 4).
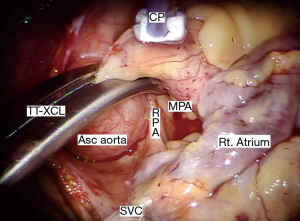
When using endo-aortic occlusion, the balloon position must be precise and remain stable in the ascending aorta (Asc aorta). There is a potential for either innominate artery balloon occlusion or intra-ventricular displacement. Therefore, TEE and bilateral arterial pressure monitoring are mandatory. This method provides effective aortic occlusion, antegrade cardioplegia, and an air vent.
Myocardial protection
When using the trans-thoracic aortic clamp method, a long cardioplegia/vent Asc aorta cannula is introduced through a pledgeted 4-0 polytetrafluoroethylene (PTFE) purse-string, which is passed either through a chest wall trocar or the working incision.
Either Custodiol™—Bretschneider’s HTK solution (Franz Köhler Chemie Bensheim GMBH, Germany) or Del Nido solution provides safe long operative periods without requiring frequent reinfusions. For maximal myocardial protection, we recommend a systemic perfusion temperature of 28 °C.
Cardiac exposure
After going on CPB, the pericardium should be opened longitudinally 3 cm anterior to the phrenic nerve. Two to three well-spaced trans-thoracic retraction sutures are placed along the inferior (dorsal) pericardial edge. Care should be taken to avoid stretching or cauterizing the phrenic nerve.
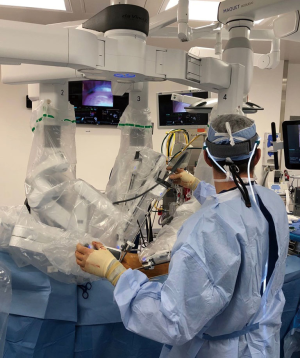
The robotic cardiac team
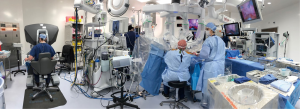
An organized team is necessary for a successful robotic cardiac surgical program. This includes anesthesiologists, surgeons, tableside assistants, scrub personnel, circulating nurses, and perfusionists (Figure 5). Team and procedure training at an experienced robotic valve center are most important before launching a RAMV program. Optimally, the team should be proficient with peripheral perfusion techniques as well as operating through minimally invasive incisions. Post-training proctoring for the first cases insures the best outcomes.
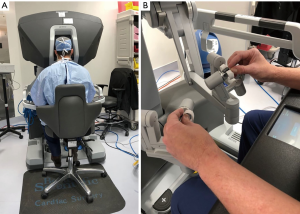
The daVinci™ surgical system
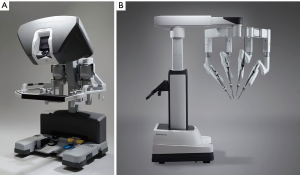
The daVinci ™ surgical system consists of an operating console, an electronic vision cart, and a surgical instrument cart. Tele-manipulators with micro-instrument end-effectors provide tremor free dexterity with both dominant and non-dominant hands (Figure 6). A clutching mechanism enables hand repositioning to maintain an optimal ergonomic attitude with respect to the visual field. We have used the daVinci™ SI HD dual console surgical system: however, the newer daVinci™ XI System (2014) (Figure 7) has the advantages of docking laser targeting as well as an extra instrument arm articulation that decreases instrument arm collisions. Both the daVinci SI™ and XI™ have dual-console capability, which enables surgeon collaboration during complex cases and facilitates training.
Instrument cart, trocar placement, and instruments
The instrument cart should be positioned along the patient’s left side with the arm activators arching over to the right chest. (Figure 8). Usually trocar placement can be camera guided through specific intercostal spaces. The most important anatomic instrument arm target, which provides the best converging annular plane trajectory, is the right superior pulmonary vein. Usually the left and right instrument arm trocars are inserted in the 3rd and 5th intercostal spaces, respectively. (Figure 9). The left trocar is positioned just superior to the anterior axillary line and the right one near the mid-axillary line. The 3D endoscopic camera is inserted either through the 4th interspace working incision, or a trocar placed anterior to it. The dynamic retractor is inserted through a mid-clavicular line trocar placed in the 5th interspace. The most common daVinci XI articulated instruments used for RAMV surgery are shown in Figure 10. These include curved scissors, Resano and DeBakey tissue forceps, large suture cut needle holders and the dynamic left atrial retractor.
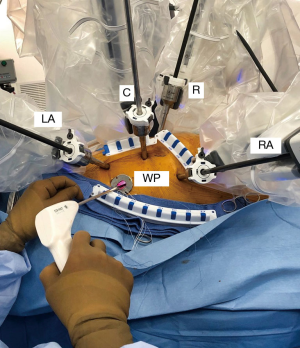
RAMV repairs
After the da Vinci™ system instruments are “trocar-docked” at the operating table, CPB is begun, and the pericardium is opened and distracted laterally. We place the aortic purse-string suture and cardioplegia cannula using robotic instruments. Modifications and simplifications of the Carpentier methods remain the mainstay of our repair strategy (5). Table 3 shows our “Technique Toolbox” that we use for our robotic mitral valve repairs.
Table 3
| Anterior leaflet prolapse | Posterior leaflet prolapse | Bileaflet prolapse (barlow) | Commissure prolapse | |
|---|---|---|---|---|
| Small segment | Large segment | |||
| Triangular resection (Small segment) | Triangular resection* | Trapezoid resection | AL = PTFE Neochords* | Commissure closure |
| PL = multiple triangular resections | Alfieri stitch or “magic stitch” | |||
| PTFE Neochords* (Large segment) | PTFE Neochords* | PTFE Neochords* | AL = PTFE Neochords* | PTFE Neochords* |
| PL = multiple folding-plasties | ||||
| Papillary folding-plasty* for multiple chords | Native chord* transfer | “Haircut” edge resection + native chord transfer or PTFE chords | AL = PTFE Neochords* | PL = sliding-plasty + PTFE Neochords |
| PL = leaflet sliding-plasty* | ||||
| Combined techniques | Leaflet folding-plasty* | Leaflet folding-plasty* | Combined techniques | Papillary folding-plasty* (elongated or multi papillary: PL and AL chords) |
| – | Inter-scallop cleft closure | Inter-scallop cleft closure | – | – |
*, a chapter illustration. AL, anterior leaflet; PTFE, polytetrafluoroethylene; PL, posterior leaflet.
Posterior leaflet repairs
For leaflet repairs, we use polyamide monofilament 4-0 Cardionyl™ sutures (Peters Surgical, Paris, France) to close resection defects. To reduce isolated scallop prolapse or chordal ruptures, we prefer triangular/trapezoidal resections (Figure 11) that do not extend to the annulus as well as placement of PTFE Neochords (Gore-Tex™-Gore, Inc. Phoenix, AZ, USA) (Figure 12). Less frequently we employ various folding-plasty and leaflet imbrication techniques for posterior leaflet repairs. In severe Barlow’s degenerative valves, we have substituted large resections and sliding-plasties, with folding-plasties, triangular resections, and PTFE neochord insertions.

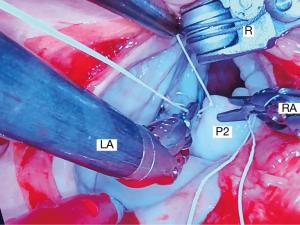
Anterior leaflet repairs
Small isolated areas of anterior leaflet prolapse, or chordal rupture also can be treated effectively by a limited triangular resection. More commonly, we prefer PTFE Neochords to reduce any anterior leaflet prolapse. To implant 4-0 PTFE Neochords one suture is passed through papillary muscle head with a second one delivered in the opposite direction, creating a wide-based “sling”. Thereafter, each suture is double-passed through the leaflet edge, adjusted and tied. Cryolife (Kennesaw, GA, USA) makes a PTFE Neochord cluster (Chord-X™) that from a single papillary muscle attachment several leaflet chords emanate (Figure 13).

Commissure repairs
For severe either P1–A1 or P3–A3 commissural prolapse, we have used commissure closure, papillary muscle shortening, and neochord implantation techniques. For limited prolapse the commissure can be imbricated. Shortening either a single or multiple papillary muscle heads can reduce simultaneously bi-leaflet (A3 and P3) posterior commissure prolapse.
Annuloplasty techniques
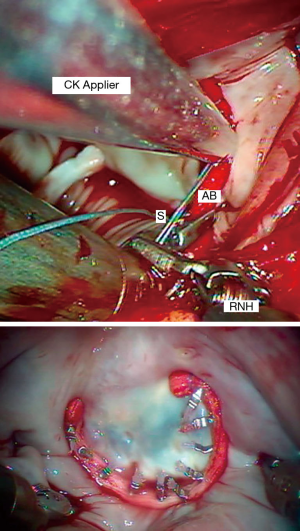
We perform an adjunctive annuloplasty in all repairs to restore the native geometry, reduce the annular size, prevent further dilatation, and reinforce the repair. We have used the Edwards Cosgrove Annuloplasty Band System™ (Edwards Lifesciences, CA, USA) for most robotic mitral repairs. Generally, a “trigone to trigone” posterior band provides optimal coaptation, while preserving a “saddle-shaped” systolic configuration. The first sutures are placed at the right fibrous trigone and then continued in a clockwise direction. We use 2-0 Ticron™ (Covidien, Mansfield, MA, USA) sutures to secure annuloplasty bands with the automated Cor-Knot™ device (LSI Solutions, Victor, NY, USA) (Figure 14). This method has reduced our cross-clamp times dramatically.
RAMV replacements
Thickened rheumatic valves can be difficult to excise as articulated robotic instruments lack the necessary force. Therefore, we excise the thick leaflet and chordal tissue manually using long instruments through a 5–6 cm working incision. Chord sparing replacements are done by placing subvalvular valve pledgeted sutures robotically. We preserve all native posterior leaflet chords by passing sutures through the leaflet edge and back through the annulus. Organizing suture guides are placed around the working incision. Thereafter, sutures are passed through the prosthetic valve sewing-ring before native annulus positioning and are secured using the Core-Knot™ technique. A ventricular vent is placed before closing the left atrium to remove residual air.
RAMV reoperations
Most of these operations have been done in patients having had prior coronary artery bypass surgery with and intact internal thoracic artery. We systemically cool these patients to 26 °C and induce ventricular fibrillation by rapid pacing using a specialized Swan Ganz catheter. In patients having Type 3b ischemic insufficiency, we use a complete Edwards ETlogix™ IMR Annuloplasty ring (Edwards Lifesciences, Inc., Irvine, CA, USA). With a symmetrically dilated posterior annulus, we either implant an Edwards Cosgrove Annuloplasty Band™. In patients with severely tethered chords from a dilated ventricle, we recommend valve replacement. When replacing a failed mitral bioprosthesis, it is best to excise it using direct vision and long instruments. In these reoperations minimal atrial retraction helps to maintain aortic valve competency.
Complications and solutions
Tables 4-6 list some of the major pitfalls, complications, and preventative measures associated with RAMV surgery. Specific complications most often are related to peripheral cardiopulmonary bypass cannulation/perfusion, aortic occlusion, and myocardial protection. Moreover, cases of unilateral reperfusion pulmonary edema have been reported by a number of centers. Some reports have shown the right lateral entry approach to be associated with increased phrenic nerve injuries (1%). The use of retrograde perfusion in minimally invasive mitral surgery has been criticized in the past. However, in patients carefully screened for peripheral vascular disease, the stroke risk with retrograde perfusion is no higher than with antegrade inflow (7,8). The Cleveland Clinic group developed a very effective screening algorithm to minimize the risk of neurologic complications (9).
Table 4
| Pitfalls | Prevention | |
|---|---|---|
| Retrograde perfusion | Aortic dissection | Preoperative vascular assessment |
| Leg ischemia | Preoperative CT with contrast | |
| Atheroemboli | Echo-guided Seldinger cannulation | |
| Monitor leg O2 saturations—femoral artery shunt | ||
| Right axillary artery cannulation | ||
| Trans-thoracic aortic clamping | Aortic injury | Preoperative assessment of aorta in patients at risk |
| PA injury | Direct or endoscopic transverse sinus visualization during clamping | |
| Left atrial appendage tear | Decrease pump flow when clamping | |
| Left main coronary injury |
PA, pulmonary artery.
Table 5
| Pitfalls | Prevention |
|---|---|
| Phrenic nerve injury | Visualization of phrenic—limited stretch—care with cryoablation |
| Cross clamp lung injury | Visualize clamp tip and lung when inserting |
| Unilateral pulmonary edema | No barotrauma—PEEP during CPB—frequent lung recruitment—limited lung manipulation |
| Residual air leak | Care when placing trans-thoracic sutures and trocars |
PEEP, positive end-expiratory pressure; CPB, cardiopulmonary bypass.
Table 6
| Pitfalls | Prevention |
|---|---|
| Inadequate myocardial protection | Frequent cardioplegia infusions—systemic cooling—no LV distension—good venous drainage |
| Coronary disease | Preoperative coronary cath/CTA |
| Aortic insufficiency | Ventricular venting |
| Pulmonary artery hypertension | Milrinone and nitric oxide therapy |
| Air embolism—cerebral and coronary | Meticulous de-airing |
| Circumflex coronary injury | No air in cardioplegia catheter |
| Meticulous annular sutures |
LV, left ventricle; CTA, coronary CT angiography.
Clinical outcomes
Our early series of over 700 minimally invasive non-robotic videoscopic mitral valve repairs had an operative mortality of 0.2% (10). Our first published robotic mitral repair report detailed 540 consecutive patients with 86 undergoing a concomitant cryo-MAZE to ablate atrial fibrillation. The 30-day mortality was 0.2% for an isolated mitral repair and 3.6% for a replacement (11). Between May 2000 and July 2014, the author performed 944 robotic mitral valve repairs with 321 of repairs having a concomitant cryomaze procedure (12). Of these patients 98% had degenerative pathology with a 100% repair rate for these. Protean degenerative mitral valve pathology required both simple and complex mitral reconstructions. Our repair “toolbox” included, among others, leaflet sliding-plasties, leaflet folding-plasties, neochord replacements, chordal transfers, papillary muscle shortening, and cleft closures as well as band annuloplasties. Immediately after the repair, 98% of patients had either no or trace mitral regurgitation by TEE. For the entire robot-assisted mitral repair series, the overall 30-day mortality was 1.4% but for patients having a repair alone was 0.15%. Follow-up of the 944 patients revealed a 2.5% overall failure rate requiring a reoperation. Of these patients 57% of patients were discharged from the hospital within 4 days. To date, our group has performed well over 1,000 robotic mitral valve repairs. Others have reported similar mortality, morbidity, and repair quality in addition to decreased perfusion and cross clamp times with experience (13-18). In a recent STS Database publication, the same results were shown in patients over 65-year old (19). Moreover, cost effectiveness for robotic mitral repair has been proven by some centers (20,21). Thus, today large volume reference centers have shown that robotic mitral valve surgery is safe, efficacious, and can provide excellent repair results as well as other patient benefits.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Peyman Sardari Nia) for the series “Minimally Invasive Mitral Valve Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.07.02). The series “Minimally Invasive Mitral Valve Surgery” was commissioned by the editorial office without any funding or sponsorship. WRCJ serves as an unpaid editorial board member of Journal of Visualized Surgery from Jun 2015 to May 2021. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The information in this publication was collected and manuscript written by the author, who verifies that it is accurate. Consent for any patient photographs was obtained through author’s global hospital operative consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chitwood WR. editor. Atlas of Robotic Cardiac Surgery. London: Springer, 2017.
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Gravlee GP, Davis RF, Hammon JW, Kussman BD. editors. Cardiopulmonary Bypass and Mechanical Support: Principles and Practice. Philadelphia: Wolters Kluwer, 2015:143-53.
- Carpentier A, Adams D, Filsoufi F. Carpentier’s Reconstructive Valve Surgery. 1st Edition. Saunders Elsevier, 2010.
- Chitwood WR Jr. Robot-assisted mitral valve repair—triangular resection. Asvide 2019;6:220. Available online: http://www.asvide.com/watch/32905
- Falk V, Cheng DC, Martin J, et al. Minimally invasive versus open mitral valve surgery: a consensus statement of the international society of minimally invasive coronary surgery (ISMICS) 2010. Innovations (Phila) 2011;6:66-76. [Crossref] [PubMed]
- Modi P, Chitwood WR Jr. Retrograde femoral arterial perfusion and stroke risk during minimally invasive mitral valve surgery: is there cause for concern? Ann Cardiothorac Surg 2013;2:E1 [PubMed]
- Gillinov AM, Mihaljevic T, Javadikasgari H, et al. Early results of robotically assisted mitral valve surgery: Analysis of the first 1000 cases. J Thorac Cardiovasc Surg 2018;155:82-91.e2. [Crossref] [PubMed]
- Modi P, Rodriguez E, Hargrove WC 3rd, et al. Minimally invasive video-assisted mitral valve surgery: a 12-year, 2-center experience in 1178 patients. J Thorac Cardiovasc Surg 2009;137:1481-7. [Crossref] [PubMed]
- Nifong LW, Rodriguez E, Chitwood WR Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg 2012;94:38-42; discussion 43. [Crossref] [PubMed]
- Chitwood WR Jr. Robotic mitral valve surgery: overview, methodology, results, and perspective. Ann Cardiothorac Surg 2016;5:544-55. [Crossref] [PubMed]
- Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: Potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e1-4.
- Suri RM, Burkhart HM, Daly RC, et al. Robotic mitral valve repair for all prolapse subsets using techniques identical to open valvuloplasty: establishing the benchmark against which percutaneous interventions should be judged. J Thorac Cardiovasc Surg 2011;142:970-9. [Crossref] [PubMed]
- Ramzy D, Trento A, Cheng W, et al. Three hundred robotic-assisted mitral valve repairs: the Cedars-Sinai experience. J Thorac Cardiovasc Surg 2014;147:228-35. [Crossref] [PubMed]
- Murphy DA, Moss E, Binongo J, et al. The Expanding Role of Endoscopic Robotics in Mitral Valve Surgery: 1,257 Consecutive Procedures. Ann Thorac Surg 2015;100:1675-81; discussion 1681-2.
- Kim HJ, Kim JB, Jung SH, et al. Clinical outcomes of robotic mitral valve repair: a single-center experience in Korea. Ann Cardiothorac Surg 2017;6:9-16. [Crossref] [PubMed]
- Gillinov AM, Suri R, Mick S, et al. Robotic mitral valve surgery: current limitations and future directions. Ann Cardiothorac Surg 2016;5:573-6. [Crossref] [PubMed]
- Wang A, Brennan JM, Zhang S, et al. Robotic mitral valve repair in older individuals: an analysis of the Society of Thoracic Surgeons Database. Ann Thorac Surg 2018;106:1388-93. [Crossref] [PubMed]
- Suri RM, Thompson JE, Burkhart HM, et al. Improving affordability through innovation in the surgical treatment of mitral valve disease. Mayo Clin Proc 2013;88:1075-84. [Crossref] [PubMed]
- Mihaljevic T, Koprivanac M, Kelava M, et al. Value of robotically assisted surgery for mitral valve disease. JAMA Surg 2014;149:679-86. [Crossref] [PubMed]
Cite this article as: Chitwood WR Jr. Robot-assisted minimally invasive mitral valve surgery. J Vis Surg 2019;5:68.