Three trocars laparoscopic Whipple’s procedure with completely intracorporeal handsewn anastomoses—case report
Introduction
Pancreatic head cancer is an aggressive malignancy with a high mortality rate and a poor prognosis. Treatment by pancreaticoduodenectomy has seen improved perioperative outcomes and complication rates over the last decades (1-3). However, it remains one of the most difficult digestive procedures to be performed, mainly because realized in front of malignant diseases with interest of vascular structures. Since its description by open surgery (4), it was described to be feasible by minimally invasive surgery (MIS) (5) and robotic-assisted laparoscopy (6). The main advantages of MIS, remain a less blood loss (7,8) and a reduced length of hospital stay (7-9), despite a longer operative time (7,9). Rate of complications between MIS and open surgery appear similar (7-9). In terms of oncologic data, the progression free survival is superior after MIS, but the overall survival is similar (8).
In the last decade, reduced port laparoscopic surgery (RPLS) has been introduced (10). Due to the difficulty to perform a laparoscopic Whipple’s procedure, usually 5–9 trocars are placed in the abdomen. Each trocar is known to be associated to a potential morbidity like bleeding, hematoma, abscess, postoperative pain and postoperative incisional hernia. Hence, RPLS was introduced to improve the cosmetic outcomes, the patient’s comfort, and to reduce the trocars’ complications, allowing to perform the same procedure as multi-trocar laparoscopy.
In front of pancreatic head cancer, like the case reported, RPLS can be applied, but a selection of the patients is required. The disease was not staged advanced and the involvement of the main vessels was absent. Then after, the patient was not submitted to a previous abdominal surgery, and the BMI was not overweight or obesity.
Preoperative evaluation/considerations
A 57-year-old man presented a first episode of cutaneous jaundice, associated to a fatigue, without history of previous surgery and without familiar history of cancer. The physical exam was negative for palpable mass in the abdomen. The first control by blood test showed an increased level of total and direct bilirubin and elevated level of Ca19.9. Hence, the patient underwent to upper gastrointestinal endoscopic ultrasound with biopsy, abdominal CT-scan and PET-CT. These exams showed an adenocarcinoma of the pancreatic head, classified as stage: cT1N0M0. After have informed the patient and explained the operation to cure the disease, a full laparoscopic Whipple’s procedure using three abdominal trocars was planned. Perioperatively, the reconstruction step was performed following the realization of the three anastomoses by intracorporeal handsewn technique.
Surgical technique (Video 1)
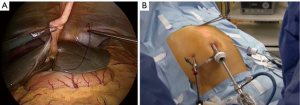
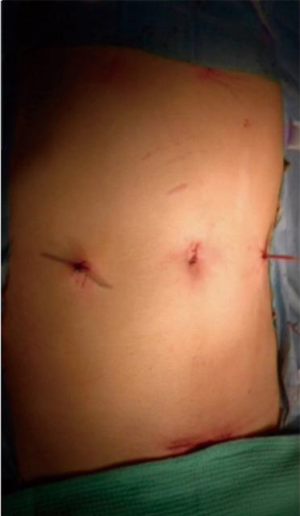
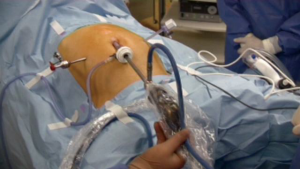
The patient was placed supine with the legs apart. A general anesthesia was induced. The umbilical scar was opened and, after have reached the peritoneal cavity, a 12-mm trocar was inserted. Two 5-mm trocars were introduced in the right and left flank respectively (Figure 1). A 10-mm 30° scope was inserted into the umbilical trocar and the peritoneal cavity as well as the hepatic surface was explored. A first percutaneous suture, using a straight needle, was placed under the xyphoid access and passed into the round ligament of the liver (Figure 2A). This suture was fixed externally by two Kelly-graspers (Figure 2B). A second percutaneous suture was inserted in a similar way in the right hypochondrium and passed in the gallbladder capsule. Cholecystectomy was retrogradely performed, with the section of the cystic duct and cystic artery between clips. The gastrocolic ligament was opened using ultrasonic shears [Lotus (laparoscopic operation by torsional ultrasound) ultrasonic scalpel (S.R.A. Developments, Ashburton, Devon, United Kingdom)] until to take down the hepatic colic flexure. A third percutaneous suture was temporally passed through the gastric antrum. The gastrocolic trunk was put in evidence and dissected between clips. The second and third duodenum were mobilized. The attachments of these latter were sectioned until to reach the hepatic pedicle posteriorly. A fourth percutaneous suture was temporally passed through the third part of the duodenum, to improve the operative field’s exposure. The third duodenum was freed using the ultrasonic shears until to put in evidence the superior mesenteric vein. Tributary veins were identified, isolated and sectioned between clips. The first jejunum was mobilized from lateral to medial. Some lymph nodes of the mesentery were found. Hence, an approach of the bowel loop from medial to lateral was considered. The first bowel mesentery was sectioned staying below these evidenced nodes, using the coagulating hook, the ultrasonic shears and clips. The posterior attachments of the second part of the duodenum were sectioned, exposing the inferior vena cava. Lymphadenectomy of the hepatic pedicle was started, sectioning the tissue under the common bile duct and exposing the portal vein. The peritoneum at the hepatic hilum was opened and the lymphadenectomy continued around the common bile duct. The dissection continued below the common bile duct. A piece of cotton tape was introduced into the abdomen through the 5-mm trocar in the right flank and passed around the common bile duct. Lymphadenectomy around the common hepatic artery was performed, using the coagulating hook laterally and posteriorly. Then, the pars flaccida of the hepatogastric ligament was sectioned. The proper hepatic artery was identified with its bifurcation. The right gastric artery was sectioned between clips. The peritoneal sheet covering the pancreas was incised at the level of the body using the coagulating hook, allowing to perform the lymphadenectomy of the celiac trunk. The splenic vein and the common hepatic artery were exposed. The gastroduodenal artery was approached from laterally and, once freed, was sectioned between clips. The lymphadenectomy was continued around the celiac trunk and the common hepatic artery, going also deeply behind this latter. At the end, the portal vein and the vessels of celiac trunk were well exposed. The isolated lymph nodes were placed into a plastic bag, introduced into the abdomen through the 12-mm umbilical trocar. The level of the gastric section was chosen and the greater and smaller curvature were freed from the correspondent ligaments. The retro-pancreatic tunnel above the superior mesenteric vein was created and the pancreas was encircled by a piece of cotton tape, at the level of chosen transection. The 10-mm scope was changed with a 5-mm long scope and introduced through the 5-mm trocar in the left flank (Figure 3). The articulating linear stapler (Frankenmann International Ltd., Sheung Wan, Hong Kong) was inserted into the 12-mm umbilical trocar and the stomach was sectioned using blue firings. The jejunum was sectioned by white firings of linear stapler. The pancreas was sectioned using the ultrasonic shears. The posterior pancreatic attachments were freed using the ultrasonic shears, staying laterally to the superior mesenteric vein and artery. The common bile duct was temporally clamped by a metallic clamp inserted through the 12-mm umbilical trocar. The common bile duct was sectioned using scissors. The specimen was removed through a plastic protection of the suprapubic access and sent for frozen pathological examinations. After have received the free margins confirmation, the reconstruction part of the procedure was started. The pancreatico-jejunostomy was firstly realized using an end-to-side handsewn anastomosis. A posterior absorbable running suture (PDS 2/0) with a preformed knot was started at the superior pancreatic edge and different bites between the pancreatic stump and the bowel were performed until to reach the inferior edge of the pancreas. Then, another running suture with a new PDS 2/0 was started at the superior pancreatic margin and, once the bowel was opened, the anterior layer of the anastomosis was completed. The hepatico-jejunostomy was confectioned in an end-to-side method using two PDS 4/0 running sutures. The temporary clamp was kept closed. After have finished the posterior running suture, the bowel was opened by the coagulating hook and the anterior layer of the anastomosis was realized with another new suture. At the end, the clamp was removed and the leak-test of the biliary anastomosis was automatically performed. The gastro-jejunostomy was realized in an end-to-side completely handsewn method. A bowel segment was chosen to reach the gastric stump easily. After have performed the posterior running suture using a PDS 1, both viscera were opened and the anterior layer was closed. Two abdominal drains were left in the abdomen close to the anastomoses. The umbilical access was closed in layers, as well as the suprapubic access (Figure 4).
The procedure ended in 6 hours 46 minutes, and operative bleeding was 600 cc.
Post-operative care
The patient was hospitalized for the first 24 hours in the intensive care unit and later in the digestive clinic. The postoperative course was uneventful and a liquid diet was started on 5th day with a solid diet on 7th day. The patient was discharged on 9th postoperative day. Hence the patient tolerated the procedure and perioperatively as well as postoperatively there wasn’t any adverse or unanticipated events.
Pathologic report confirmed a well differentiated pancreaticobiliary adenocarcinoma, with perinervous infiltration and lymphovascular emboli, free margins, 0 metastatic lymph nodes on 24 isolated, and 8° UICC edition stage: pT1cN0.
After multidisciplinary counseling, a decision to submit the patient to adjuvant chemotherapy was taken. At 18 months of follow-up the patient is doing well and free of disease (Figure 5).
Conclusions
Laparoscopic Whipple’s procedure remains an advanced procedure to be performed by laparoscopy. Besides the other advantages of MIS, this technique can add in selected patients a reduced trocar’s morbidity and an improved patient’s comfort.
Acknowledgments
This video was presented as podium presentation at the Annual Clinical Congress of the American College of Surgeons (ACS), San Francisco, October 27–31, 2019.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs.2019.12.09/coif). GD serves as an unpaid editorial board member of Journal of Visualized Surgery from June 2019 to May 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sun H, Ma H, Hong G, et al. Survival improvement in patients with pancreatic cancer by decade: a period analysis of the SEER database, 1981-2010. Sci Rep 2014;4:6747. [Crossref] [PubMed]
- Basson JJ, Du Toit RS, Nel CJ. Carcinoma of the head of the pancreas. Morbidity and mortality of surgical procedures. S Afr J Surg 1994;32:9-12. [PubMed]
- Zovak M, Mužina Mišić D, Glavčić G. Pancreatic surgery: evolution and current tailored approach. Hepatobiliary Surg Nutr 2014;3:247-58. [PubMed]
- Whipple AO. Pancreaticoduodenectomy for Islet Carcinoma: A Five-Year Follow-Up. Ann Surg 1945;121:847-52. [Crossref] [PubMed]
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [Crossref] [PubMed]
- Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 2010;24:1646-57. [Crossref] [PubMed]
- Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg 2012;215:810-9. [Crossref] [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg 2014;260:633-8; discussion 638-40. [Crossref] [PubMed]
- Song KB, Kim SC, Hwang DW, et al. Matched case-control analysis comparing laparoscopic and open pylorus-preserving pancreaticoduodenectomy in patients with periampullary tumors. Ann Surg 2015;262:146-55. [Crossref] [PubMed]
- Mori T, Dapri G (eds). Reduced port laparoscopic surgery. Tokyo: Springer Japan, 2014.
- Dapri G, Bascombe N, Montorsi M. Three trocars laparoscopic Whipple. Asvide 2020;7:074. Available online: http://www.asvide.com/watch/33113
Cite this article as: Dapri G, Bascombe N, Montorsi M. Three trocars laparoscopic Whipple’s procedure with completely intracorporeal handsewn anastomoses—case report. J Vis Surg 2020;6:31.