Complete thymectomy for myasthenia gravis
Introduction
Myasthenia gravis (MG) is a relatively rare autoimmune disorder with different severities of muscle weakness. Although it can be life-threatening in some cases, MG is generally treatable and even curable with multidisciplinary treatments (1). In 1912, Schumacher and Roth first reported an improvement of myasthenic weakness after thymus removal for the treatment of thyrotoxicosis in an 18-year-old female patient (2). Later in 1939, Blalock et al. noticed a durable remission of generalized MG in a 24-year-old woman after thymectomy for the treatment of thymic cystic tumor (3). Two years later, Blalock described encouraging early results of six patients with MG after the removal of the thymus gland and recommended total thymectomy to alter the course of MG (4). Following these inspiring preliminary investigations, many surgeons performed numbers of observational retrospective studies to evaluate the role of thymectomy in the treatment of MG (5,6). In 2016, the first randomized controlled trial evaluating thymectomy in MG patients seropositive for anti-acetylcholine receptor (anti-AChR) autoantibody demonstrated that significant benefits in the improvement of neurological outcome and steroid-sparing effect favors extended transsternal thymectomy within a three-year follow-up (7). Recently, the 5-year results from MGTX still favor thymectomy for these patients (8). It is now common knowledge that thymectomy is an indispensable and effective option for patients with MG.
The use of the da Vinci surgical system in thymectomy for the treatment of MG was first reported by Ashton et al. in 2003 (9). Since then many institutes have adopted robotic technology for thymectomy and reported their experience and clinical outcomes of patients undergoing robotic thymectomy for MG (10-13). Up to now, many approaches for robotic thymectomy have been developed and described, including unilateral, bilateral and subxiphoid (11,14-16). With the 3-dimensional (3-D) visualization, high dexterity and enhanced precision provided by the da Vinci surgical system, robotic thymectomy has been proved not only to be safe and feasible (17-21), but also to provide MG patients with encouraging neurological outcomes even comparing with the extended transsternal thymectomy (19-21).
Although controversies exist as to the best approach for robotic thymectomy in the treatment of MG patients, we have been performing robotic thymectomy via a left-sided unilateral approach for MG patients since 2003 and consider it a perfect combination of minimal invasiveness and maximal exposure for resection. Furthermore, it could be shown that left-sided robotic extended thymectomy is significantly beneficial for MG patients according to cumulative complete remission comparing with non-robotic thoracoscopic thymectomy (21). Herein, we describe the left-sided robotic extended thymectomy for MG patients in detail, including patient selection and workup, preoperative preparation, equipment preference cart, procedure and tips, tricks and pitfalls.
Patient selection and workup
The main indications of robotic extended thymectomy for MG are patients with resectable thymomas or patients without thymomas aiming to reduce or avoid the use of immunosuppressive medications (22). The diagnosis of MG is usually confirmed by relevant symptoms and a positive test for antibodies against acetylcholine receptors (AChR), muscle-specific kinase (MuSK), and lipoprotein receptor-related protein 4 (LRP4) (23). If the antibodies are negative, neurophysiological tests and a Tensilon test would be performed to confirm the diagnosis (24). In anti-MuSK and anti-LRP4 MG patients, previous studies have shown that thymectomy has little effects on the improvement of MG symptoms if any (22,25). Although early removal of the thymus seems to be beneficial for MG patients (26,27), consensus and guidelines recommend that thymectomy should be performed after MG is well controlled to avoid postoperative respiratory failure (22,28).
Furthermore, a computed tomography (CT) scan of the chest should be performed in each MG patient before thymectomy, and if there is a mass with soft-tissue attenuation, contrast-enhanced CT will be performed to assess the enhancement characteristics and the adjacent structures of the lesion (29). If the lesion is adherent to adjacent tissues like major vessels, phrenic nerves or the sternum, the robotic extended thymectomy will not be suitable. In differentiating the hyperplasia from the thymoma, magnetic resonance imaging (MRI) can be better than CT, as thymoma may sometimes show diffuse thymic enlargement and may be diagnosed as hyperplasia in CT scan (30). Positron emission tomography-CT (PET-CT) can help to differentiate thymic cancer from other thymic diseases, but overlapping uptake value exists between normal and abnormal findings (31). Both MRI and PET-CT can be performed in need, but not routinely. In addition, pulmonary function tests and routine blood examinations for surgery as well as the electrocardiogram should also be used in the assessment of MG patients before the surgery.
Preoperative preparation
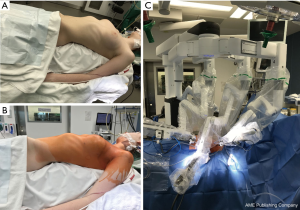
The position of the three trocars is usually marked on the skin for each case on the ward. Our usual approach for robotic extended thymectomy is performed through the left side under general anesthesia with a left-sided double-lumen endotracheal tube. Before the operation, the patient is placed supine and moved to the left edge of the operating table, the left arm is placed lower than the table plane, providing enough space for the motion of the instruments (Figure 1A). Thereafter, the operative field is prepared, sterilized and draped, exposing the adequate area to make sure a conversion to open or an introduction of a right-sided trocar could be easily performed if necessary (Figure 1B). Then, the 8mm trocar, to which the 30-degree robotic camera is mounted, is inserted at the 4th intercostal space on the midaxillary line, followed by insufflating carbon dioxide (CO2) to pressure about 8 mmHg. However, the insufflation of CO2 should be used only for the placement of the resting trocars or even be avoided if possible so that an exact incision of the mediastinal pleura can be performed. Subsequently, the 8mm cranial and caudal special working trocars are inserted at the 3rd intercostal space in the anterior axillary line and the 5th intercostal space between the anterior axillary line and the middle clavicular line respectively. The patient cart with four arms is docked on the right side of the patient (Figure 1C). The surgeon console is positioned at some distance from the patient while the vision cart is placed at the bottom of the operating table.
Equipment preference cart
Robotic extended thymectomy was performed initially with the basic model of the da Vinci system, later on refined accordingly with the development of the further models of the S-, and Si-system, and is actually performed with the da Vinci system Xi (Intuitive Surgical, Sunnyvale, CA), the fourth generation of the da Vinci system, which consists of a surgeon console, a patient cart with four robotic arms and a 3-D vision system. Comparing to the former Si and X generations, the Xi system has smaller, thinner arms, longer instrument shafts, allowing a greater range of motion and greater operational reach. Besides, the computerized automatic positioning system can help to shorten the docking process. Three robotic arms are connected with the surgical instruments, the middle one holding the camera, the cranial and the caudal ones connecting with an ultrasonic dissector and a precise bipolar forcep, respectively, they can wrist and rotate much more dexterously than human hands. The surgeon sits at the console with his fingers grasping the master controls to operate with extreme precision.
Procedure
We present here a complete process of robotic assisted thymectomy (Video 1).
The positioning of the trocars always follows a rule of measuring adapted to the very different anatomy of the patients and to the sternum. The initial use of CO2-insufflation may be useful or even necessary at this point. The next step is the inspection of the thymic gland and the straight view at the left phrenic nerve with the 30° optic looking down. For MG patients with thymoma, meticulous inspection of the tumor is necessary before any dissection to re-check if the thymoma is adherent to any major vessels, nerves or adjacent organs. Furthermore, thoracoscopic exploration should be made to exclude or prove stage IV in thymoma cases. During the operation, the “no-touch” surgical technique should be obeyed to make sure the tumor is not directly manipulated, minimizing the risk of tumor seeding. Furthermore, according to the International Thymic Malignancy Interest Group criteria (32), the surgical margins should be assessed by the pathologist with microscopic inspection after an en bloc resection of the thymus, thymoma and the mediastinal and lower cervical fat.
The dissection usually starts at approximately the middle of the pericardium along the left phrenic nerve where the thymus gland is regularly visible. In elder and/or obese cases where this area is frequently covered by fat tissue, the CO2 insufflation can be applied to get a better view. There is always a gap between the upper part of the main thymic lobe – even if covered by large amounts of fat and the lower cardiophrenic fatty tissue.
The dissection is extended caudally along the left phrenic nerve until the phrenic bundle is isolated and the tissue in the aortopulmonary window is completely mobilized. Care should be taken of the left phrenic nerve while isolating, in some cases, the thymus gland can extend across the nerve or completely include the nerve. Dissection is continued upwards along the nerve until the cervical pleura is reached at the entrance to the innominate vein.
Then, the pleural incision is extended up to the jugular fold of the mediastinal pleura. Further dissection of the neck area at the left phrenic nerve allows for visualizing the innominate vein, regularly medial to the left phrenic nerve. Thereafter, the pressure of CO2 can be increased to get a better view for the dissection of the neck area. However, attention should be paid to the hemodynamic monitoring.
After that, mobilization of the upper poles of the thymus is continued above the left innominate vein, but sometimes the anatomical variations of the upper poles do exist, e.g., the upper poles run behind or around the innominate vein. The upper poles are mobilized by a combination of blunt dissection shifting the tissue with open branches of the grasping instruments (long bipolar or Maryland dissector). Intermediate grasping, mechanical and harmonic dissection add to the isolating of the thyrothymic ligament where both of the poles are divided.
The upper poles are managed by grasping and bringing them down to get a complete resection. They are divided bluntly with spread branches of the grasping instrument by ultrasonic dissection from the inferior portion of the thyroid gland.
In most cases, two to four thymic veins are collecting all the venous blood into the innominate vein. Thymic veins are served with a harmonic scalpel without using clip ligatures. It is worth mentioning that anatomical variations of thymic veins should be always kept in mind, albeit rare.
Subsequently, the dissection of the cervical pleural fold is continued to the median retrosternal line. The right lung is visualized coved only by the mediastinal pleural which is kept closed. After taking down the right upper pole, the right mammary vein is found. At its entrance into the venous confluence, the right phrenic nerve will be presented at the lateral side of the superior vena cava. Following the nerve along the vena cava down allows for complete dissection of the tissue in the aortocaval groove. On the right side, continue the dissection towards the subxiphoid pleural fold until the right lung is visible. The right phrenic nerve can be frequently found at the root of the right lung. Then mobilize the tissue block from the pericardium. Attention should be paid to keep the right pleural cavity closed here, otherwise, the effect of CO2 insufflation on enlarging the operative field will be lost.
The whole median retrosternal tissue is mobilized, then the right thymic lobe is visualized with surrounding fat tissue in the aortocaval groove. Then, the tissue in the left cardiophrenic angle is dissected and continue upwards. The last dissection proceeds from the left cardiophrenic angle to the subxiphoid area and extends to the right cardiophrenic area. The right pleura is opened at last and the cardiophrenic tissue is dissected completely.
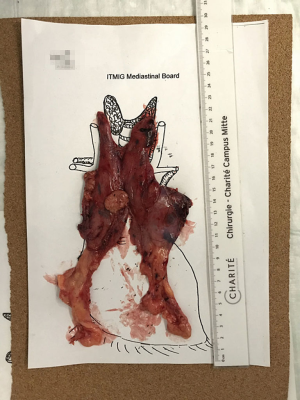
In conclusion, the en bloc resected thymus specimen, including the mediastinal fat and thymoma (if any), is removed out in an endo bag through the camera incision. The whole specimen is weighed immediately after the removal, then is placed on a mediastinal board as in situ and photographed before sending it to the pathologists (Figure 2).
Role of team members
The surgical team usually consisted of a board-certified console surgeon, a board-certified table surgeon, an anesthesiologist, a scrub nurse, and an operating room nurse. The console surgeon controlled the robot to perform an extended thymectomy. The table surgeon, on the left side of the patient, should excel at inserting the trocars and connecting, adjusting and changing the surgical instruments, also have a thorough knowledge of managing emergencies like major bleeding that may require a conversion to transsternal thymectomy. A scrub nurse should be familiar with the robotic materials and qualified for the adjustment and change of the robotic instruments. An operating room nurse should know how to connect and calibrate the robotic components. An experienced anesthesiologist in this team was supposed to be familiar with the management of both robotic-assisted thoracic surgery and the general anesthesia in MG patients.
Each member of the robotic thymectomy team is crucial for the success of the robotic extended thymectomy. They should learn more than just professional knowledge but also be educated to be familiar with the da Vinci system, in case any technical problems occur during the procedure.
Tips, tricks, and pitfalls
The proper approach for robotic thymectomy has been an ongoing debate for decades among thoracic surgeons worldwide. Some surgeons supposed it was dangerous doing the left-sided approach for thymectomy, concerning about the indeed reports of cardiac injuries with trocar insertion. But this can be perfectly avoided by visual control guided the trocar insertion with scissors and adequate CO2 insufflation at the beginning. In fact, more than 900 cases have been performed in the Charité without any pericardium or heart injury during the placement of trocars. The insufflated CO2 can help to enlarge the space at the anterior mediastinum, providing adequate space for trocar placement. On the other hand, using stitch scissors with round tips to open the intercostal space by blunt dissection and to guide the insertion of the trocars can make sure the insertion is under control all the time. Furthermore, we have introduced this method into many institutes where the surgeons are doing quite well.
Another main reason why many surgeons favor the right-sided approach is that the visualization of the superior vena cava is beneficial for identifying the left innominate vein at the venous confluence. However, through the left-sided approach, the left innominate vein can also be easily identified after opening the cervical pleura fold at the point medial to the phrenic nerve. Before dissecting the upper poles from the inferior portion of the thyroid gland, grasping and pulling them downward is always necessary for a radical resection, and in this way, the overall anatomy of the left innominate vein is exposed, then identifying and dissecting the thymic veins which drain into the left innominate vein is followed without too much difficulty.
The authors prefer left-sided approach for robotic thymectomy, not only because the left lobe of the thymus is usually larger, but also because it is easy to handle the upper poles, manage the thymic veins, approach to the contralateral phrenic nerve and access the common anatomical locations with ectopic thymic tissue from the left side. The thymic tissue can extend to or even across the left phrenic nerve in many cases, which can hardly be addressed from the right side (33). Sometimes, the upper poles can hide behind or run circularly around the innominate vein, which is easier to manage through the left-sided approach. Besides, the left innominate vein mainly runs on the left side, which provides benefits for managing the thymic veins as well as protecting the innominate vein. Recently, our team did a close review on the presence of ectopic thymic tissue in patients undergoing thymectomy for MG (34), the results showed some preferred anatomical locations of ectopic thymic tissue: anterior mediastinal fat, pericardiophrenic angles, aortopulmonary window, cervical region (pretracheal fat) and lateral to phrenic nerves (mostly at the left side). Importantly, our previous study has shown that an extended thymectomy favors the left-sided approach (35).
During the last few years, subxiphoid robotic thymectomy has attracted increasing attention worldwide (16,36). This procedure offers surgeons a direct visualization of the mediastinum similar to median sternotomy in a minimally invasive way. Although the operative view of the neck region is good, when it comes to the anatomical variation of the upper poles running behind or even surround the innominate vein and ectopic thymic tissue behind the left innominate vein, the left approach could be more direct. Besides, long-term follow-up data are needed to explicit the neurological outcomes of MG.
International consensus and guidelines recommend all MG patients with resectable thymoma undergo thymectomy after MG is well controlled, and thymectomy is performed for the treatment of thymoma with the resection of thymus and the mediastinal and lower cervical fat (22,28,37). Although many studies have shown robotic thymectomy is safe and feasible for MG patients with early-stage thymoma, the proper size of thymoma for robotic thymectomy and the long-term oncologic outcome after robotic thymectomy are still under debate (38-40). Available data has shown encouraging early and mid-term oncologic outcomes in MG patients with thymoma after robotic thymectomy (21,41). As for the proper size of thymoma for robotic thymectomy, although no international consensus or guideline is dictating the size of thymoma as a criterion for robotic thymectomy, most surgeons consider thymomas smaller than 5 cm are proper and acceptable for robotic thymectomy (38,42,43). However, recent studies have shown that robotic thymectomy is also safe and feasible for thymomas larger than 5cm and the perioperative outcomes of patients are better when compared with open surgery (44,45). The authors also had some experience in handling large-sized thymomas in a robotic-assisted way, usually, a fourth trocar would be introduced at the subxiphoid to assist exposure and to take out the specimen. Thus, the authors recommend that robotic extended thymectomy is indicated in all patients with resectable thymoma, typically Masaoka Koga I and II.
High cost has long been considered as a pitfall for robotic surgery, also docking and undocking time needed for the relatively newly established team, especially when an emergency occurs. Since the console surgeon is not able to handle the emergency immediately due to the non-sterilized situation, there should always be an experienced surgeon sitting by the patient during the robotic surgery. Besides, conversion to open should be performed if major bleeding occurs or the oncologic principles (including incomplete resection, not en bloc resection or perforation of the capsule) are violated. Systemic training is needed for the whole team in the OR to avoid the unexpected accident, and a contingency plan should always be prepared before the robotic-assisted surgery.
Conclusions
The robotic approach is perfect for application to thymectomy, wherein the operative space is confined and the working angles are difficult for human hands. The left-sided robotic extended thymectomy is safe and feasible with encouraging clinical outcomes for both MG and thymoma. However, further researches with long-term follow up is still required before the robotic approach becomes the gold standard for thymectomy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Jean-Marc Baste) for the series “Robotic Assisted Thoracic Surgery: Advanced Procedures in Lung and Mediastinum: From Post-induction TTT (immunotherapy) to Sleeve Resection, Complex Segmentectomies and Extended Thymectomy for Myasthenia Gravis” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs.2020.03.08/coif). The series “Robotic Assisted Thoracic Surgery: Advanced Procedures in Lung and Mediastinum: From Post-induction TTT (immunotherapy) to Sleeve Resection, Complex Segmentectomies and Extended Thymectomy for Myasthenia Gravis” was commissioned by the editorial office without any funding or sponsorship. MI serves as an unpaid editorial board member of Journal of Visualized Surgery from Jun 2019 to May 2021. JCR reports and declares as a proctor for Intuitive Surgical. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gilhus NE. Myasthenia Gravis. N Engl J Med 2016;375:2570-81. [Crossref] [PubMed]
- Schumacher E, Roth J. Thymektomie bei einem fall von morbus basedowii mit myasthenie Med Chir 1912;25:746. (in German).
- Blalock A, Mason MF, Morgan HJ, et al. Myasthenia gravis and tumours of the thymic region - Report of a case in which the tumor was removed. Ann Surg 1939;110:544-61. [Crossref] [PubMed]
- Blalock A, Harvey AM, Ford FR, et al. The treatment of myasthenia gravis by removal of the thymus gland - Preliminary report. J Amer Med Assoc 1941;117:1529-33. [Crossref]
- Keynes G. The results of thymectomy in myasthenia gravis. Br Med J 1949;2:611-6. [Crossref] [PubMed]
- Perlo VP, Poskanzer DC, Schwab RS, et al. Myasthenia gravis: evaluation of treatment in 1,355 patients. Neurology 1966;16:431-9. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol 2019;18:259-68. [Crossref] [PubMed]
- Ashton RC Jr, McGinnis KM, Connery CP, et al. Totally endoscopic robotic thymectomy for myasthenia gravis. Ann Thorac Surg 2003;75:569-71. [Crossref] [PubMed]
- Rea F, Marulli G, Bortolotti L. Robotic video-assisted thoracoscopic thymectomy. Multimed Man Cardiothorac Surg 2005;2005:mmcts 2004 000422.
- Rückert JC, Ismail M, Swierzy M, et al. Thoracoscopic thymectomy with the da Vinci robotic system for myasthenia gravis. Ann N Y Acad Sci 2008;1132:329-35. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Wetscher G, et al. First experiences with the da Vinci operating robot in thoracic surgery. Eur J Cardiothorac Surg 2004;25:844-51. [Crossref] [PubMed]
- Fleck T, Fleck M, Muller M, et al. Extended videoscopic robotic thymectomy with the da Vinci telemanipulator for the treatment of myasthenia gravis: the Vienna experience. Interact Cardiovasc Thorac Surg 2009;9:784-7. [Crossref] [PubMed]
- Goldstein SD, Yang SC. Assessment of Robotic Thymectomy Using the Myasthenia Gravis Foundation of America Guidelines. Ann Thorac Surg 2010;89:1080-5; discussion 1085-6. [Crossref] [PubMed]
- Kawaguchi K, Fukui T, Nakamura S, et al. A bilateral approach to extended thymectomy using the da Vinci Surgical System for patients with myasthenia gravis. Surg Today 2018;48:195-9. [Crossref] [PubMed]
- Suda T, Tochii D, Tochii S, et al. Trans-subxiphoid robotic thymectomy. Interact Cardiovasc Thorac Surg 2015;20:669-71. [Crossref] [PubMed]
- Orsini B, Santelmo N, Pages PB, et al. Comparative study for surgical management of thymectomy for non-thymomatous myasthenia gravis from the French national database EPITHOR. Eur J Cardiothorac Surg 2016;50:418-22. [Crossref] [PubMed]
- Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [PubMed]
- Renaud S, Santelmo N, Renaud M, et al. Robotic-assisted thymectomy with Da Vinci II versus sternotomy in the surgical treatment of non-thymomatous myasthenia gravis: Early results. Rev Neurol 2013;169:30-6. [Crossref] [PubMed]
- Cakar F, Werner P, Augustin F, et al. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur J Cardiothorac Surg 2007;31:501-4. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016;87:419-25. [Crossref] [PubMed]
- Zisimopoulou P, Brenner T, Trakas N, et al. Serological diagnostics in myasthenia gravis based on novel assays and recently identified antigens. Autoimmun Rev 2013;12:924-30. [Crossref] [PubMed]
- Chiou-Tan FY, Gilchrist JM. Repetitive nerve stimulation and single-fiber electromyography in the evaluation of patients with suspected myasthenia gravis or Lambert-Eaton myasthenic syndrome: Review of recent literature. Muscle Nerve 2015;52:455-62. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Massa R, et al. Long-term outcome of thoracoscopic extended thymectomy for nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg 2009;36:164-9. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Lerut TE, et al. Thoracoscopic thymectomy in autoimmune myasthenia: Results of left-sided approach. Ann Thorac Surg 2000;69:1537-41. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y, Suzuki Y, et al. Delayed remission after thymectomy for myasthenia gravis of the purely ocular type. J Thorac Cardiovasc Surg 1996;112:371-5. [Crossref] [PubMed]
- Sussman J, Farrugia ME, Maddison P, et al. Myasthenia gravis: Association of British Neurologists' management guidelines. Pract Neurol 2015;15:199-206. [Crossref] [PubMed]
- Priola AM, Priola SM. Imaging of thymus in myasthenia gravis: from thymic hyperplasia to thymic tumor. Clin Radiol 2014;69:e230-45. [Crossref] [PubMed]
- Inaoka T, Takahashi K, Mineta M, et al. Thymic hyperplasia and thymus gland tumors: differentiation with chemical shift MR imaging. Radiology 2007;243:869-76. [Crossref] [PubMed]
- Sadato N, Tsuchida T, Nakaumra S, et al. Non-invasive estimation of the net influx constant using the standardized uptake value for quantification of FDG uptake of tumours. Eur J Nucl Med 1998;25:559-64. [Crossref] [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Mulder DG, White K, Herrmann C Jr. Thymectomy. Surgical procedure for myasthenia gravis. AORN J 1986;43:640-6. [Crossref] [PubMed]
- Li F, Tao Y, Bauer G, et al. Unraveling the role of ectopic thymic tissue in patients undergoing thymectomy for myasthenia gravis. J Thorac Dis 2019;11:4039-48. [Crossref] [PubMed]
- Rückert JC, Czyzewski D, Pest S, et al. Radicality of thoracoscopic thymectomy--an anatomical study. Eur J Cardiothorac Surg 2000;18:735-6. [Crossref] [PubMed]
- Zhang H, Chen L, Zheng Y, et al. Robot-assisted thymectomy via subxiphoid approach: technical details and early outcomes. J Thorac Dis 2018;10:1677-82. [Crossref] [PubMed]
- Kadota Y, Horio H, Mori T, et al. Perioperative management in myasthenia gravis: republication of a systematic review and a proposal by the guideline committee of the Japanese Association for Chest Surgery 2014. Gen Thorac Cardiovasc Surg 2015;63:201-15. [Crossref] [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [Crossref] [PubMed]
- Qian L, Chen X, Huang J, et al. A comparison of three approaches for the treatment of early-stage thymomas: robot-assisted thoracic surgery, video-assisted thoracic surgery, and median sternotomy. J Thorac Dis 2017;9:1997-2005. [Crossref] [PubMed]
- Seong YW, Kang CH, Choi JW, et al. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur J Cardiothorac Surg 2014;45:e68-73; discussion e73. [Crossref] [PubMed]
- Keijzers M, Dingemans AM, Blaauwgeers H, et al. 8 years' experience with robotic thymectomy for thymomas. Surg Endosc 2014;28:1202-8. [Crossref] [PubMed]
- Toker A, Erus S, Ozkan B, et al. Does a relationship exist between the number of thoracoscopic thymectomies performed and the learning curve for thoracoscopic resection of thymoma in patients with myasthenia gravis? Interact Cardiovasc Thorac Surg 2011;12:152-5. [Crossref] [PubMed]
- Schwartz GS, Yang SC. Robotic thymectomy for thymic neoplasms. Thorac Surg Clin 2014;24:197-201. vii. [Crossref] [PubMed]
- Kneuertz PJ, Kamel MK, Stiles BM, et al. Robotic Thymectomy Is Feasible for Large Thymomas: A Propensity-Matched Comparison. Ann Thorac Surg 2017;104:1673-8. [Crossref] [PubMed]
- Wilshire CL, Vallieres E, Shultz D, et al. Robotic Resection of 3 cm and Larger Thymomas Is Associated With Low Perioperative Morbidity and Mortality. Innovations 2016;11:321-6. [Crossref] [PubMed]
Cite this article as: Rückert JC, Zhang H, Li F, Uluk D, Ismail M, Meisel A. Complete thymectomy for myasthenia gravis. J Vis Surg 2021;7:8.