
Narrative review: open surgery for thoracoabdominal aortic aneurysm—is it still a horrible surgery?
Introduction
Thoracoabdominal aortic aneurysm (TAAA) is defined as a dilatation of the aorta that is at least 1.5 times the diameter of nearby aortic segments (1). Like all aneurysms, TAAAs are repaired to prevent life-threatening rupture. Aortic pathology is complex and may be related to several histopathologic conditions including medial degeneration, atherosclerosis, aortic dissection, genetic disorders, and vasculitis. Most patients with TAAA have no symptoms attributable to their disease at the time of diagnosis, which usually stems from an incidental finding on computed tomography. The most common initial symptom in patients with TAAA is vague pain, which can occur in the chest, back, flank, or abdomen. The American Heart Association (AHA) has issued guidelines for diagnosing and managing aortic disease, which includes recommendations regarding the history and physical examination for patients with suspected thoracic aortic disease (2).
Nonoperative management, which consists of strict blood pressure control, cessation of smoking, and at least yearly surveillance with imaging studies, is appropriate for asymptomatic patients who have small aneurysms, which will continue to slowly grow in size. Typically, patients undergo elective repair once the aneurysm meets a diameter-based threshold. As per the guidelines, TAAA repair in asymptomatic patients is indicated if the aortic diameter exceeds 6 centimeters, if the aortic diameter exceeds 5.5 centimeters in patients with chronic aortic dissection, or if the rate of dilation is more than 1 cm/year. In patients with Marfan syndrome or a related heritable thoracic aortic disease, the diameter-based threshold for operation is lower. In patients with symptoms, especially pain, or with complications of a superimposed acute dissection, the risk of impending rupture warrants expeditious evaluation and urgent aneurysm repair, even when the above-mentioned threshold diameters have not been reached. Possible signs and symptoms of rupture include sudden sharp pain that radiates to the back, a palpable and pulsatile mass in the abdomen, hypotension, loss of consciousness, and shortness of breath. Repair of TAAA is typically performed in patients in their mid-60s, and common comorbidities include pulmonary disease, coronary artery disease, and chronic kidney disease (3).
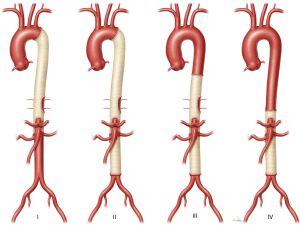
The repair of TAAAs necessitates exposing the aorta above and below the diaphragm and must incorporate the segment of the aorta containing the visceral arteries—the celiac axis, the superior mesenteric artery (SMA), and both renal arteries. These procedures are separated into four classes as determined by their location on the thoracoabdominal aorta (Figure 1). The Crawford classification of TAAA repairs enables appropriate risk stratification and selection of treatment modalities based on the extent of the aortic replacement (4). Crawford extent I repairs commonly involve the aorta from near the left subclavian artery to the celiac trunk; these repairs may involve the renal arteries, but they do not extend into the infrarenal aorta. Crawford extent II repairs commonly involve nearly the entire distal aorta, typically spanning the aorta between the left subclavian artery to the infrarenal aorta and often to the aortoiliac bifurcation. Crawford extent III repairs tend to involve the region between the mid-descending thoracic aorta (below the 6th rib) and a variable portion of the abdominal aorta. Finally, Crawford extent IV repairs begin within the diaphragmatic hiatus and extend to the abdominal aorta. In extents II-IV, aortic repair may extend into the iliac arteries. Generally, Crawford extent II TAAA repairs carry the greatest surgical risk because they are the most extensive repair (5,6). We present the following article in accordance with the Narrative Review reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-113/rc).
Contemporary adjuncts
The modern era of surgical treatment of TAAA began in 1974 when E. Stanley Crawford published a small series of repairs showing significant reductions in mortality (7). Dr. Crawford applied the Dacron graft in a new way by using an anatomically based in-situ repair that involved patch reattachment for branching arteries, rather than earlier methods, which relied on an extra-anatomical approach and individual grafts to reattach each branching artery. Over time, Crawford moved from a clamp-and-sew approach to explore the use of left heart bypass (LHB), renal perfusion, an early form of cerebrospinal fluid (CSF) drainage, and other techniques. The publication of his lifetime surgical experience highlighted an impressive early mortality rate of 8% (8). However, for extent II repairs, spinal cord deficits (paraplegia and paraparesis) remained a challenging problem with an incidence of 31%.
Based on clinical studies and the lessons learned over the last decades, our approach has evolved to a multimodal approach designed to reduce complications and improve outcome (Table 1). Basically, the multimodal approach involves the surgical repair of TAAA using several adjunctive techniques such as mild passive hypothermia, LHB, sequential cross-clamping, reimplantation of intercostal and lumbar arteries, selective visceral perfusion and cold perfusion of renal arteries, and CSF drainage during and after surgery. Adjunctive techniques are used based on the extent of repair; because extent II TAAA repair replaces the largest segment of the aorta and carries the greatest operative risk, adjuncts are used most extensively during this repair. Implementing these adjuncts improves patient outcomes and reduces the risks of open repair. Because of generally reduced mortality and morbidity rates, various multimodal approaches have been widely used in managing TAAAs (Table 2). Repair in hemodynamically unstable patients may preclude the use of protective adjuncts.
Table 1
| All Crawford extents of TAAA repair |
| Mild passive hypothermia (32–34 ℃) |
| Moderate heparinization (1 mg/kg) |
| Aggressive reattachment of intercostal and lumbar arteries (especially between T8 and L1) |
| Selective use of branched aortic grafts (presence of connective tissue disorder or visceral and renal artery ostias are apart) |
| Sequential aortic clamping (when possible) |
| Cold renal perfusion (4 ℃) when renal ostia are accessible |
| Crawford extent I and II TAAA repairs and select other repairs |
| Cerebrospinal fluid drainage |
| Left heart bypass (during proximal anastomosis) |
| Selective perfusion of celiac axis and superior mesenteric artery |
| Certain extensive or highly complex aortic repairs |
| Hypothermic circulatory arrest |
| Elephant trunk completion or reversed elephant trunk repair |
| Extraction, full salvage, or partial salvage of prior endovascular repair |
TAAA, thoracoabdominal aortic aneurysm.
Table 2
| Author, year | All repairs (n) | Extent II TAAA repairs, n (%) | Use of HCA | Use of LHB | Use of renal perfusion | Use of CSF drainage |
|---|---|---|---|---|---|---|
| Tanaka, 2019, (9) | 2,012a | 329 (16.4) | Rare | Routine | Routine (cc) | Routine |
| Shimamura, 2019, (10) | 393 | 196 (49.9) | None | Routine | Routine (cc) | Routine |
| Latz, 2019, (11) | 516 | 100 (19.4) | None | Routine | Routine (cc) | Routine |
| Kouchoukos, 2019, (12) | 285 | 107 (37.5) | Routine | None | None | Routine |
| Girardi, 2017, (13) | 711b | 96 (13.5) | Rare | Selective | Routine (pls) | Routine |
| Wynn, 2016, (14) | 805c | 200 (24.8) | Rare | None | Routine (cc) | Routine |
| Murana, 2016, (15) | 542 | 285 (52.6) | Rare | Routine | Routine (cc) | Routine |
| Coselli, 2016, (3) | 3,309 | 1,066 (33.4) | Rare | Selective | Routine (cc) | Selective |
| Tshomba, 2014, (16) | 84 | 25 (29.8) | None | Selective | Routine (cc/HKT) | Selective |
a, mixed series of descending thoracic (n=681) and thoracoabdominal (n=1,331) aortic aneurysm repairs; b, mixed series of descending thoracic (n=229) and thoracoabdominal (n=482) aortic aneurysm repairs; c, mixed series of descending thoracic (n=89) and thoracoabdominal (n=716) aortic aneurysm repairs. Rare is defined as an infrequently used approach, such as the use of HCA only when the proximal aorta cannot be safely clamped. Routine is defined as a preferred approach that is used in the majority of patients when possible, such as when surgical exposure permits the insertion of perfusion catheters. Selective is defined as an approach that is used in certain circumstances, such as a technique being reserved for Crawford extents I and II TAAA repair. TAAA, thoracoabdominal aortic aneurysm; HCA, hypothermic circulatory arrest; LHB, left heart bypass; CSF, cerebrospinal fluid; cc, cold crystalloid; pls, plasmalyte; HKT, histidine-tryptophan-ketoglutarate.
Mild hypothermia
To provide end organ and spinal cord protection, the patient’s temperature is permitted to drift down during the procedure, and the core temperature routinely reaches 32–34 ℃. Rarely, in patients with very large or ruptured aneurysms that preclude proximal clamping of the aorta, we use hypothermic circulatory arrest; however, other aortic centers use this method routinely (12).
Left heart bypass
We use LHB, or distal aortic perfusion, in all extent I and II repairs and selectively use it in lesser extents (extents III and IV). Using LHB provides perfusion to the spinal cord and visceral organs during the proximal anastomosis. Studies have been conducted to examine the role of LHB in reducing the risk of paraparesis or paraplegia. Our retrospective review of patients with extent I and II repairs demonstrated that LHB provided the most benefit for extent II repairs (17). The rate of paraplegia and paraparesis was significantly lower in patients in whom the repair was performed with LHB than in those performed without LHB (4.8% vs. 13.1%, P=0.007, extent II TAAA repairs) (17). Our results demonstrating the benefits of LHB in select TAAA groups are consistent with the findings of other large studies (18,19).
Spinal cord protection
Spinal cord protection in TAAA repair is a multimodal approach using several adjunct techniques discussed above, including LHB, sequential aortic cross-clamping, mild hypothermia, and heparinization, and the modalities specific to the spinal cord discussed in this section. To study the efficacy of CSF drainage in extensive repairs, we compared the outcomes in 76 patients undergoing extent I or II repairs with CSF drainage (pressure goal of 10 mmHg or lower from the onset of the procedure to 48 hours post-procedure) with those of 69 patients undergoing the same procedures without CSF drainage as part of a randomized clinical trial (20). Although 30-day mortality was similar between the groups, the rate of paraplegia or paraparesis was significantly higher in the control group than in the CSF drainage group (13.0% versus 2.6%, respectively; P=0.03). As such, current AHA/ACC guidelines recommend the use of CSF drainage in patients with an increased risk of spinal injury (2). We routinely use CSF drainage to help decrease rates of paraplegia and paraparesis. CSF drainage decreases the intraspinal pressure by draining fluid, which increases blood flow to the spinal cord.
Another important way to protect the spinal cord is to sustain perfusion of the spinal cord via reattached intercostal or lumbar arteries during and after aortic surgery. In a study of 1,114 extent II repairs, reattachment of at least one pair of intercostal or lumbar arteries protected against persistent paraplegia or paraparesis, with a relative risk reduction of 0.38 (P<0.001) (21). However, reattaching intercoastal and lumbar arteries to prevent spinal cord complications is a controversial topic. According to the Griepp collateral network concept, because of the presence of collateral vascular networks perfusing the thoracolumbar spinal cord, these segmental aortic vessels can be sacrificed during TAAA repair without neurologic deficits (22). Because the reattachment of these segmental arteries is a fairly simple process and our analysis of Crawford extent II repairs showed such a reduction in the risk of paraplegia (21), we follow an aggressive reattachment strategy (especially between T8 and L1).
Renal protection
To protect kidneys during aortic cross-clamping, we use mild hypothermia, LHB, and selective renal hypothermia with cold renal perfusion. One of the most effective ischemic protection techniques is hypothermia. In our randomized controlled trial of 30 patients undergoing extent II repair, cold crystalloid (lactated Ringer’s solution), infused at 4 ℃, was protective against acute kidney injury when compared with normothermic blood (23). Other studies support this conclusion (24). In a second trial, we compared cold blood and cold crystalloid renal perfusion as modalities to protect kidneys during extensive TAAA repair in 172 patients. We found that resultant early death, renal failure requiring hemodialysis, and serum renal biomarkers were similar between the two groups; however, no spinal cord deficits were seen in the cold crystalloid perfusion group, whereas the use of cold blood resulted in 5 patients with deficits (25). Although our preference has been to use cold crystalloid as a renal perfusate, we are now exploring the use of a histidine-tryptophan-ketoglutarate solution as a renal perfusate.
Visceral protection
Intestines can tolerate longer ischemic times than the kidneys before irreversible damage develops. However, the intestinal mucosa is highly susceptible to ischemia resulting from both local and remote organ dysfunction. Therefore, efforts to limit intestinal ischemia are important aspects of TAAA repair (26,27). General strategies to reduce visceral organ ischemia and maximize visceral organ perfusion include mild hypothermia, sequential aortic clamping, and LHB to provide distal aortic perfusion. Selective visceral perfusion, using isothermic blood, is provided after LHB is discontinued by rerouting the LHB circuit. Furthermore, every effort is made to limit vessel ischemic times—operative techniques include giving priority to visceral artery anastomoses after intercostal artery reattachment, using beveled anastomosis techniques (whenever possible), and avoiding hemodynamic instability during and after surgery. Since a significant proportion of patients with TAAA have concomitant visceral occlusive disease, to improve blood flow through occluded visceral arteries, endarterectomy may be performed, or balloon-expandable stents may be used (28).
Surgical technique
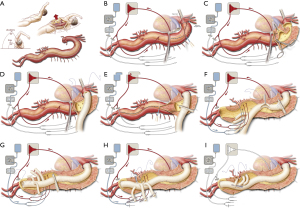
Our surgical approach to open repair of TAAAs has been described previously (5,29). In brief, a sigmoid-shaped skin incision is made from behind the left scapula, along the 7th rib, across the costal margin, and toward the left periumbilical region (Video 1, Figure 2). The chest is entered through the 6th intercostal space. The entire thoracoabdominal aorta is exposed by performing medial visceral rotation and by a circumferential division of the diaphragm. After heparin (1.0 mg/kg) is administered, a cannula is placed in the left atrium via a left inferior pulmonary venotomy and connected to the drainage line of the LHB circuit. Another cannula is placed in the distal descending thoracic aorta and connected to the inflow line of the circuit. After LHB flow is initiated, the proximal aortic clamp is typically placed just distal to the left subclavian artery, and another distal aortic clamp is applied across the mid-descending thoracic aorta. The isolated segment of the proximal descending thoracic aorta is then longitudinally opened, and the aorta is completely divided at the level of the proximal anastomosis. The graft material of choice for aortic surgery is Dacron impregnated with either collagen or gelatin. Usually, a 22-, 24-, or 26-mm graft is used. After structural anastomoses are completed, pledgeted mattress sutures may be placed strategically around each anastomosis to reduce the risk of bleeding after unclamping. Once the proximal anastomosis is completed, LHB is discontinued. In the presence of chronic dissection, the dissecting membrane between the true and false lumen is completely excised. Then, whenever the ostia of the renal arteries are accessible, 9-French balloon perfusion catheters are placed in the renal arteries to infuse them with cold perfusate. Selectively, the SMA and the celiac axis are perfused with isothermic blood via 9-French balloon perfusion catheters from the rerouted LHB circuit. At least one pair of large intercostal arteries with slow back bleeding is attached as a small patch to the opening in the side of the graft (Video 1). After this stage, another opening is made in the side of the graft adjacent to the visceral branches. Commonly, the celiac, SMA, and right renal arteries are reimplanted together as a patch, and the left renal artery is reattached by direct reimplantation or via a small-diameter graft (8- or 10-mm); options for reimplantation include a larger 4-vessel patch as well as the use of additional small diameter grafts, including the use of commercially available 4-branched grafts. To treat dissection extending into the visceral arteries, the dividing septum is fenestrated, excised, sutured closed, or obliterated with a small balloon-expandable stent (28). As needed, visceral arteries are managed with endarterectomy, balloon-expandable stents, or bypass grafts. If the visceral arteries are especially fragile or significantly displaced from each other or if the patient has heritable thoracic aortic disease, a 4-branched graft may be used (30). During extent II, III, and IV repairs, the distal anastomosis is usually constructed at the level of the aortic bifurcation or, occasionally, to each iliac or femoral artery separately.
Outcomes
Open repair of TAAA has respectable outcomes and remains the only treatment approved by the United States Food and Drug Administration. This notion is supported by our study of 3,309 TAAA open repairs; operative death occurred in 249 patients (7.5%), permanent paraplegia in 97 patients (2.9%), renal failure requiring dialysis in 189 patients (5.7%), and gastrointestinal ischemia in 31 patients (0.9%) (3). When patients were divided into subgroups, operative death was nearly doubled in patients undergoing emergency repair as compared to elective repair (12.2% vs. 6.2%, P<0.001). As expected, morbidity and mortality were higher for extent II repairs than for extent I, III, or IV repairs because of the complexity and magnitude of the intervention. The outcomes of some patient groups bear special mention. Generally, patients undergoing open TAAA repair under the age of 50 (31), those with heritable thoracic aortic disease (such as Marfan syndrome) (32,33), and patients with chronic aortic dissection (rather than those with degenerative aneurysm) (34) had more favorable outcomes than their counterparts. Other contemporary studies have also demonstrated favorable outcomes for open repair of TAAA (Table 3); in several of these studies, the early death rate was below 8% (3,10-13,16).
Table 3
| Author, year | All repairs (n) | Early death, n (%) | Paraplegiaa, n (%) | Stroke, n (%) | Renal failure, n (%) | Durability estimate |
|---|---|---|---|---|---|---|
| Tanaka, 2019, (9) | 2,012b | 316 (15.7) | 167 (8.3)a | 109 (5.4) | 432 (21.5) | Not available |
| Shimamura, 2019, (10) | 393 | 24 (6.1) | 19 (4.8) | Not available | 18 (4.6) | Not available |
| Latz, 2019, (11) | 516 | 40 (7.8) | 60 (11.6)a | Not available | 37 (7.2) | 90% at 10 y |
| Kouchoukos, 2019, (12) | 285 | 21 (7.4) | 15 (5.3)a | 12 (4.2) | 11 (3.9) | Not available |
| Girardi, 2017, (13) | 711c | 40 (5.6) | 4 (0.6) | 27 (3.8) | 38 (5.3) | Not available |
| Wynn, 2016, (14) | 805d | 66 (8.2) | 21 (2.6) | Not available | 5 (0.6) | Not available |
| Murana, 2016, (15) | 542 | 59 (10.9) | 23 (4.2) | 23 (4.2) | 23 (4.2) | 80.7% at 10 y |
| Coselli, 2016, (3) | 3,309 | 249 (7.5) | 97 (2.9) | 74 (2.2) | 189 (5.7) | 94.1% at 15 y |
| Tshomba, 2014, (16) | 84 | 5 (5.9) | 14 (16.7)a | 2 (2.4) | 2 (2.4) | Not available |
a, includes cases of paraparesis as indicated; b, mixed series of descending thoracic (n=681) and thoracoabdominal (n=1,331) aortic aneurysm repairs; c, mixed series of descending thoracic (n=229) and thoracoabdominal (n=482) aortic aneurysm repairs; d, mixed series of descending thoracic (n=89) and thoracoabdominal (n=716) aortic aneurysm repairs. Renal failure aims to include the need for new onset dialysis that persists at discharge but may include general renal dysfunction. Durability is defined as freedom from reoperation or freedom from repair failure. TAAA, thoracoabdominal aortic aneurysm.
Open TAAA repair has been shown to be durable. In our study of 3,309 TAAA repairs, the freedom from repair failure of repair was 94% at 15 years (3). Latz and colleagues reported similar findings of 90% freedom from reintervention at 10 years (11). In general, there appears a low risk of late graft infection; however, patients with heritable thoracic aortic disease are prone to late patch aneurysms and pseudoaneurysms (33,35). Our study comparing 726 reoperative and 2,653 non-reoperative TAAA repairs found no difference in early operative risk (35).
Emerging endovascular approaches
Thoracic endovascular aortic aneurysm repair (TEVAR) and abdominal endovascular aneurysm repair (EVAR) have emerged to dominate traditional open aortic repair. However, these approaches have some important limitations. For example, standard TEVAR and EVAR cannot be used as designed in branched areas of the aorta, thus excluding their routine use in the aortic arch and the region of the abdominal aorta that gives rise to visceral vessels. However, in the last decade, significant improvements in endovascular technology including branched or fenestrated grafts have allowed for experimental endovascular repair of TAAAs; clinical trials are currently underway to study the efficacy of this approach. In previous trials of endovascular TAAA repair, the devices were custom made for the patient; however, newer endovascular devices currently being tested are available off-the-shelf. Recently, a cohort study of multibranched endograft implantation in 146 patients (only 25% of which were extensive TAAA repairs) showed a 4.8% early mortality rate, an 8.2% rate of reintervention for occlusive complications of branching arteries, and a 76.8% rate of freedom from aneurysm-related death or reintervention 5 years (36). In a population-based study comparing endovascular (n=303) and open (n=361) TAAA repair, endovascular repair was associated with early benefits (decreased rates of early mortality, length of stay, and postoperative adverse events); however, late reintervention was increased after endovascular repair, and there was no difference in long-term survival and adverse events (37).
Endovascular aortic repair is widely viewed as being less durable than open aortic repair—this is largely due to relatively high reintervention rates, especially in patients with heritable thoracic aortic disease. Although the majority of secondary procedures can be performed endovascularly, the most serious complications necessitate conversion to open repair. In our study of 67 endovascular-to-open conversions, the conversion was commonly necessitated by expanding aneurysm, infection, fistula, and rupture. For patients without infection, the rate of early mortality was similar to our other series. However, when repair was complicated by infection, early mortality was elevated (38). Stent-graft extraction, in a patient with infection, is shown in Video 2.
Conclusions
Open TAAA repair is among the most technically complex surgical procedures. Although open TAAA repairs will be challenged by emerging endovascular approaches, open repair remains the superior approach for subsets of patients. These patients are generally younger (<50 years) and have heritable thoracic aortic disease or chronic aortic dissection. Other groups of patients, such as octogenarians, face increased operative risk after open TAAA repair (39) and may have better outcomes with an endovascular approach. Over the years, contemporary techniques for open repair of TAAAs have been modified to include multiple adjuncts that improve patient outcomes. For many patient groups, these modifications have resulted in significant benefit and support the continued need for the open approach, making it far from a horrible surgery.
Acknowledgments
The authors thank the Section of Scientific Publications at the Texas Heart Institute, Susan Y. Green, MPH, Hiruni Amarasekara, MS, Ginger Etheridge, BA, and Elizabeth Akpan-Smart, MS for providing editorial support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Di Bartolomeo, Davide Pacini and Mohamad Bashir) for the series “The 10th Postgraduate Course on ‘Surgery of the Thoracic Aorta’ in Bologna” published in Journal of Visualized Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-113/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-113/coif). The series “The 10th Postgraduate Course on ‘Surgery of the Thoracic Aorta’ in Bologna” was commissioned by the editorial office without any funding or sponsorship. JSC reports to participate in clinical studies with and/or consults for Terumo Aortic, Medtronic, W.L. Gore & Associates, CytoSorbents, and Abbott Laboratories and receives royalties and grant support from Terumo Aortic, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). The manuscript is waived from patient informed consent according to the ethics committee or institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Santilli JD, Santilli SM. Diagnosis and treatment of abdominal aortic aneurysms. Am Fam Physician 1997;56:1081-90. [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. [PubMed]
- Coselli JS, LeMaire SA, Preventza O, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg 2016;151:1323-37. [Crossref] [PubMed]
- Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg 1986;3:389-404. [Crossref] [PubMed]
- Coselli JS, de la Cruz KI, Preventza O, et al. Extent II thoracoabdominal aortic aneurysm repair: How I do it. Semin Thorac Cardiovasc Surg 2016;28:221-37. [Crossref] [PubMed]
- Coselli JS, LeMaire SA, Conklin LD, et al. Morbidity and mortality after extent II thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 2002;73:1107-15; discussion 1115-6. [Crossref] [PubMed]
- Crawford ES. Thoraco-abdominal and abdominal aortic aneurysms involving renal, superior mesenteric, celiac arteries. Ann Surg 1974;179:763-72. [Crossref] [PubMed]
- Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg 1993;17:357-68; discussion 368-70. [Crossref] [PubMed]
- Tanaka A, Leonard SD, Sandhu HK, et al. Open descending and thoracoabdominal aortic repairs in patients younger than 50 years old. Ann Thorac Surg 2019;108:693-9. [Crossref] [PubMed]
- Shimamura J, Oshima S, Ozaki K, et al. Open thoracoabdominal aortic aneurysm repair: Contemporary outcomes for 393 elective cases. Ann Thorac Surg 2019;107:1326-32. [Crossref] [PubMed]
- Latz CA, Cambria RP, Patel VI, et al. Durability of open surgical repair of type I-III thoracoabdominal aortic aneurysm. J Vasc Surg 2019;70:413-23. [Crossref] [PubMed]
- Kouchoukos NT, Kulik A, Haynes M, et al. Early outcomes after thoracoabdominal aortic aneurysm repair with hypothermic circulatory arrest. Ann Thorac Surg 2019;108:1338-43. [Crossref] [PubMed]
- Girardi LN, Ohmes LB, Lau C, et al. Open repair of descending thoracic and thoracoabdominal aortic aneurysms in patients with preoperative renal failure. Eur J Cardiothorac Surg 2017;51:971-7. [Crossref] [PubMed]
- Wynn M, Acher C, Marks E, et al. The effect of intercostal artery reimplantation on spinal cord injury in thoracoabdominal aortic aneurysm surgery. J Vasc Surg 2016;64:289-96. [Crossref] [PubMed]
- Murana G, Castrovinci S, Kloppenburg G, et al. Open thoracoabdominal aortic aneurysm repair in the modern era: results from a 20-year single-centre experience. Eur J Cardiothorac Surg 2016;49:1374-81. [Crossref] [PubMed]
- Tshomba Y, Kahlberg A, Melissano G, et al. Comparison of renal perfusion solutions during thoracoabdominal aortic aneurysm repair. J Vasc Surg 2014;59:623-33. [Crossref] [PubMed]
- Coselli JS, LeMaire SA. Left heart bypass reduces paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 1999;67:1931-4; discussion 1953-8. [Crossref] [PubMed]
- Safi HJ, Hess KR, Randel M, et al. Cerebrospinal fluid drainage and distal aortic perfusion: reducing neurologic complications in repair of thoracoabdominal aortic aneurysm types I and II. J Vasc Surg 1996;23:223-8; discussion 229. [Crossref] [PubMed]
- Schepens MA, Vermeulen FE, Morshuis WJ, et al. Impact of left heart bypass on the results of thoracoabdominal aortic aneurysm repair. Ann Thorac Surg 1999;67:1963-7; discussion 1979-80. [Crossref] [PubMed]
- Coselli JS, LeMaire SA, Koksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: results of a randomized clinical trial. J Vasc Surg 2002;35:631-9. [Crossref] [PubMed]
- Coselli JS, Green SY, Price MD, et al. Spinal cord deficit after 1114 extent II open thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg 2019;S0022-5223(19)30352-6.
- Griepp RB, Griepp EB. Spinal cord perfusion and protection during descending thoracic and thoracoabdominal aortic surgery: the collateral network concept. Ann Thorac Surg 2007;83:S865-9; discussion S990-2. [Crossref] [PubMed]
- Köksoy C, LeMaire SA, Curling PE, et al. Renal perfusion during thoracoabdominal aortic operations: cold crystalloid is superior to normothermic blood. Ann Thorac Surg 2002;73:730-8. [Crossref] [PubMed]
- Svensson LG, Coselli JS, Safi HJ, et al. Appraisal of adjuncts to prevent acute renal failure after surgery on the thoracic or thoracoabdominal aorta. J Vasc Surg 1989;10:230-9. [Crossref] [PubMed]
- Lemaire SA, Jones MM, Conklin LD, et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:11-9; discussion 19. [Crossref] [PubMed]
- Aftab M, Coselli JS. Reprint of: Renal and visceral protection in thoracoabdominal aortic surgery. J Thorac Cardiovasc Surg 2015;149:S130-3. [Crossref] [PubMed]
- Waked K, Schepens M. State-of the-art review on the renal and visceral protection during open thoracoabdominal aortic aneurysm repair. J Vis Surg 2018;4:31. [Crossref] [PubMed]
- LeMaire SA, Jamison AL, Carter SA, et al. Deployment of balloon expandable stents during open repair of thoracoabdominal aortic aneurysms: a new strategy for managing renal and mesenteric artery lesions. Eur J Cardiothorac Surg 2004;26:599-607. [Crossref] [PubMed]
- Ouzounian M, LeMaire SA, Weldon S, et al. Open Repair of Thoracoabdominal Aortic Aneurysm: Step-by-Step. Operative Techniques in Thoracic and Cardiovascular Surgery 2018;23:2-20. [Crossref]
- de la Cruz KI, LeMaire SA, Weldon SA, et al. Thoracoabdominal aortic aneurysm repair with a branched graft. Ann Cardiothorac Surg 2012;1:381-93. [PubMed]
- Coselli JS, Amarasekara HS, Green SY, et al. Open Repair of Thoracoabdominal Aortic Aneurysm in Patients 50 Years Old and Younger. The Annals of Thoracic Surgery 2017;103:1849-57. [Crossref] [PubMed]
- Coselli JS, Green SY, Price MD, et al. Results of open surgical repair in patients with Marfan syndrome and distal aortic dissection. Ann Thorac Surg 2016;101:2193-201. [Crossref] [PubMed]
- Frankel WC, Song HK, Milewski RK, et al. Open Thoracoabdominal Aortic Repair in Patients with Heritable Aortic Disease in the GenTAC Registry. Ann Thorac Surg 2020;109:1378-84. [Crossref] [PubMed]
- Coselli JS, Green SY, Zarda S, et al. Outcomes of open distal aortic aneurysm repair in patients with chronic DeBakey type I dissection. J Thorac Cardiovasc Surg 2014;148:2986-93.e1-2.
- Coselli JS, Rosu C, Amarasekara HS, et al. Reoperative surgery on the thoracoabdominal aorta. J Thorac Cardiovasc Surg 2018;155:474-485 e1. [Crossref] [PubMed]
- Walker J, Kaushik S, Hoffman M, et al. Long-term durability of multibranched endovascular repair of thoracoabdominal and pararenal aortic aneurysms. J Vasc Surg 2019;69:341-7. [Crossref] [PubMed]
- Rocha RV, Lindsay TF, Austin PC, et al. Outcomes after endovascular versus open thoracoabdominal aortic aneurysm repair: A population-based study. J Thorac Cardiovasc Surg 2021;161:516-27.e6. [Crossref] [PubMed]
- Spiliotopoulos K, Preventza O, Green SY, et al. Open descending thoracic or thoracoabdominal aortic approaches for complications of endovascular aortic procedures: 19-year experience. J Thorac Cardiovasc Surg 2018;155:10-8. [Crossref] [PubMed]
- Aftab M, Songdechakraiwut T, Green SY, et al. Contemporary outcomes of open thoracoabdominal aortic aneurysm repair in octogenarians. J Thorac Cardiovasc Surg 2015;149:S134-S141. [Crossref] [PubMed]
Cite this article as: Kothari R, Weldon SA, Koksoy C, Coselli JS. Narrative review: open surgery for thoracoabdominal aortic aneurysm—is it still a horrible surgery? J Vis Surg 2022;8:4.


