
Retroperitoneal schwannoma mimicking a pancreatic cystic neoplasm: a case report
Introduction
Schwannomas are rare, slow-growing, neurogenic tumors that arise from Schwann cells of the peripheral nerve fibers (1). They are usually located in the head/neck regions or the extremities and only rarely arise in the retroperitoneal space (1,2). Retroperitoneal schwannomas (RPS) pose significant diagnostic challenges as they are frequently elusive and lack specific signs and symptoms. They have been associated with atypical presentations such as abdominal distension, hypertension, hematuria and hydronephrosis in several previously published reports (3-5). Although preoperative computed tomography (CT) and magnetic resonance imaging (MRI) may help establish the diagnosis, RPS lack specific imaging features which makes definitive diagnosis inevitably based on post-resection histopathologic evaluation and immunohistochemical labeling (5).
Herein we present a case of RPS with cystic degeneration mimicking a pancreatic cystic neoplasm. We highlight the importance of novel molecular techniques that aid in the diagnosis of this rare neoplasm. We present the following case in accordance with the CARE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-165/rc).
Case presentation
A 76-year-old male was diagnosed with an incidental pancreatic cystic lesion during workup for hematuria. He denied any symptoms and his physical examination was unremarkable. Endoscopic ultrasound (EUS) evaluation revealed an anechoic lesion that seemed to arise from the uncinate process of the pancreas measuring 2.8 cm × 1.8 cm (Figure 1A). The cyst was composed of a single compartment without septae, lacked a solid component and was not in obvious communication with the pancreatic duct. Several other smaller cystic lesions were seen throughout the pancreas. On fine needle aspiration (FNA), golden serous fluid was aspirated. Cytology revealed scant epithelial cells with no evidence of malignancy. The amylase level in the cystic fluid was 67 U/L and the carcinoembryonic antigen (CEA) level was 493 ng/mL. Molecular analysis utilizing amplification-based targeted next generation sequencing (NGS) (PancreaSeqV1) revealed no genetic alteration in key pancreatic-cancer related genes (6). On imaging, the cyst measured 2.1 cm ×1.8 cm. Given the aforementioned findings, a pancreatic cystic neoplasm was the most plausible diagnosis. As the patient was asymptomatic and the cyst lacked any worrisome features, annual follow-up was recommended.
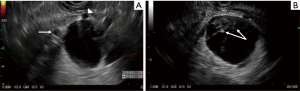
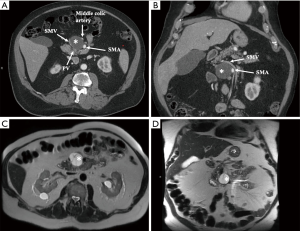
On follow-up EUS 2 years after the initial diagnosis, the cyst grew significantly in size (4.5 cm × 4.1 cm in greatest dimension). It was also noted to be compartmentalized by thick septations with internal debris (Figure 1B). On tri-phasic CT scan (Figure 2A,B) and MRI (Figure 2C,D), the growing cyst seemed to originate from the uncinate process as an exophytic outgrowth and was abutting the superior mesenteric artery and vein, as well as their proximal branches (middle colic artery and first jejunal vein, respectively). Given the likely mucinous histology posing a risk of future dedifferentiation in the setting of progressive growth, and the unfavorable location intertwined between the mesenteric vasculature, resection over continued close surveillance was advised.
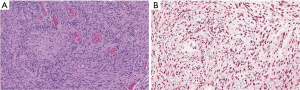
On exploratory laparotomy, the cystic lesion was seen to be arising from the retroperitoneum and was only abutting the pancreas. Furthermore, no evidence of direct invasion into adjacent visceral structures or the mesenteric vasculature was identified, thus complete enucleation was accomplished. On final histopathology evaluation (Figure 3A,B) clusters of compact elongated spindle cells were seen and immunohistochemical (IHC) stains revealed positive SOX10, weakly positive S100, and negative chromogranin and CK20. On targeted NGS analysis (OncoMineTM) (7), a genetic mutation involving both NF2 (p.G161Dfs*13) and SMARCB1 (p.R377Hc), and a copy number loss at NF2 22q12.2 were identified. These aforementioned findings support the diagnosis of a cystic peripheral nerve sheath tumor (schwannoma).
The patient had an uneventful postoperative course and was discharged home in a stable condition. He was seen in the clinic one week postoperatively and was doing well.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Oral informed consent was obtained from the patient as he was not physically available to provide written consent due to the COVID-19 pandemic.
Discussion
Schwannomas are benign peripheral nerve sheath tumors that arise from differentiated Schwann cells most commonly in the extremities and rarely in the retroperitoneum (1-3). In our case, the schwannoma mimicked a cystic neoplasm arising from the pancreatic parenchyma. Due to the progressive growth, critical anatomic location, and the inherent risk of future dedifferentiation of—what was thought to be—a pancreatic cystic neoplasm, surgical resection was performed. A cystic degeneration of a retroperitoneal schwannoma is a rare entity, and this diagnosis can be insidious and elusive requiring a high index of suspicious.
We initially performed a EUS guided FNA and applied a pancreas targeted NGS assay (PancreaSeqV1) capable of detecting genes known to be frequently mutated in pancreatic cystic lesions (KRAS, GNAS, TP53, PIK3CA, etc.) (6) which revealed the absence of any genomic alterations. Nevertheless, the clinical characteristic and the high level of CEA in the FNA fluid suggested the presence of a mucinous lesion, while the lack of mutation on PancreaSeqV1 was rationalized as a possible false negative. In retrospect, the elevated CEA level could have been obtained from one of the adjacent intrapancreatic cystic lesions that was probably sampled along the needle track.
Postsurgical histopathology evaluation by IHC was positive for S100 and SOX10 and lacked staining for chromogranin which is highly suggestive for a schwannoma. Ultimately, genomic analysis of the excised surgical specimen was performed using OncomineTM—a comprehensive targeted NGS assay that analyses 161 genes including those that have been shown to be mutated in a subset of schwannomas tumors such as NF2 and SMARCB1—thus ascertaining the presence of a peripheral nerve sheath tumor (7,8).
Although rarely, peripheral nerve sheath tumors can arise in the retroperitoneum and can have an insidious presentation. The findings presented here describe an atypical presentation of a cystic schwannoma and employ novel molecular techniques to improve diagnostic accuracy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-165/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-165/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goh BK, Tan YM, Chung YF, et al. Retroperitoneal schwannoma. Am J Surg 2006;192:14-8. [Crossref] [PubMed]
- Song JY, Kim SY, Park EG, et al. Schwannoma in the retroperitoneum. J Obstet Gynaecol Res 2007;33:371-5. [Crossref] [PubMed]
- Holbrook C, Saleem N. Retroperitoneal schwannoma: an unusual cause of abdominal distention. BMJ Case Rep 2017;2017:bcr2017220221. [Crossref] [PubMed]
- Vijayan SK, Shetty S, Bhat SR, et al. Retroperitoneal schwannoma: an atypical presentation. J Clin Diagn Res 2014;8:ND22-3. [PubMed]
- Zhang L, Gao M, Zhang T, et al. Surgical management of retroperitoneal schwannoma complicated with severe hydronephrosis: A case report. Medicine (Baltimore) 2018;97:e12528. [Crossref] [PubMed]
- Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018;67:2131-41. [Crossref] [PubMed]
- Rhodes DR, Yu J, Shanker K, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 2004;6:1-6. [Crossref] [PubMed]
- Abdellatif E. Schwannoma. PathologyOutlines.com website. Available online: http://www.pathologyoutlines.com/topic/softtissueschwannoma.html. Accessed October 20, 2020.
Cite this article as: AlMasri S, Nassour I, Singhi AD, Zureikat A, Paniccia A. Retroperitoneal schwannoma mimicking a pancreatic cystic neoplasm: a case report. J Vis Surg 2021;7:33.


