
How I do VATS segmentectomy: the uniportal approach
Introduction
Pulmonary segmentectomy is a surgical technique designed to spare healthy parenchyma. It is generally offered for diagnostic and treatment purposes in cases of centrally located nodules or metastases as well as for peripheral early-stage non-small cell lung cancer (NSCLC) (1,2). Over the past decades, resections of lesser than lobar extent (including wedge resections or segmentectomies) were merely seen as a secondary surgical option for patients who were unfit for lobectomy due to an overly diminished lung function (3,4). Similarly, it was offered to elderly patients, a population in whom segmentectomy is correlated with lower complication rates (5). Recently, the concept of lung resections with curative intent and their extent gained renewed attention for the management of pulmonary malignancies. Two main reasons underlie this development. These are the new and improved detection techniques for small nodules or ground glass opacities compatible with early-stage lung cancer and the recent improvements of minimally invasive techniques for lobectomies and segmentectomies. Both aspects rekindled the controversy on the surgical indication for segmentectomies: are they suitable for intentional resections in patients with early-stage lung cancer or only for those patients with limited lung function? During the 2021 American Association for Thoracic Surgery (AATS) annual meeting, Professor Asamura presented the results of the first randomized trial assessing lobectomy against limited-extent resection by segmentectomy for small-sized peripheral NSCLCs (JCOG0802/WJOG4607L). This is the first phase 3 trial demonstrating the benefit of segmentectomy through a significant difference in overall survival compared to lobectomy. Accordingly, segmentectomy, instead of lobectomy, should be the standard surgical procedure for patients with small sized (<2 cm, CTR >0.5) peripheral c-stage IA NSCLC (1). In addition, a similar rate of complications was reported in a multicenter study that compared complications between video-assisted thoracoscopic surgery (VATS) intentional segmentectomies and lobectomies. It also showed that the chest tube duration and the length of stay were shorter in the VATS segmentectomy group (6).
Since 2010, the concomitant development of uniportal VATS (UVATS) as a suitable alternative to multiport VATS for pulmonary anatomical resections (7,8) allowed us to gradually transition from a standard 3 port approach (launched in 2014) to UVATS (mastered in 2017). This step was accompanied by several technical achievements (9). Initially, we had to learn how to avoid or minimize instrument clutter/conflict to gain acceptable retraction and exposure through one, single utility incision with parallel and coaxial devices (10). This also included appropriate management of the stapler direction whilst maintaining proper visibility of the targeted organs, no small feat when there is only one entry port. It turns out that small incremental steps, such as the use of a bent (30º) high-resolution thoracoscope and of specific instruments, could be combined to achieve large improvements to the operative technique. UVATS has been associated with potential several advantages including less pain from fewer intercostal space incisions, better cosmetic aspect, reduced morbidity, lesser immunochemokine disturbance and accelerated functional recovery when compared to conventional MVATS (11). However, high level evidence, particularly in terms of randomized trials, is lacking and the launch of such demanding technique should be gradually and safely introduced.
To this day, segmentectomies remain a surgical challenge, especially so when they are carried out by UVATS. They require one-by-one dissection of segmental broncho-vascular structures and unequivocal identification of intersegmental planes (ISP). Both will go a long way in preventing post-operative complications due to potentially incomplete resections. Pulmonary segmentectomies can be separated into two groups: simple and complex segmentectomies, depending on whether the surgeon needs to separate one (simple segmentectomies) or multiple (complex segmentectomies) ISPs (12,13). Simple segmentectomies include the left upper lobe tri-segmentectomy (S1+2+3) or lingulectomy (S4+5), and the lower lobe apical segmentectomy (S6) or basal pyramid segmentectomy (S7+8+9+10). Complex segmentectomies include mono- or bi-segmentectomies of the upper, middle or lower lobes. In this last group the broncho-vascular structures are deeply located in the lung structures and often display large anatomical variations (14,15).
Hence, it was logical for us to proceed with simple segmentectomies first during the initial phase of multiport approach. After 25 uneventful simple cases, complex segmentectomies were gradually phased in to reach the top of the learning curve after around 80 cases. Then, we safely introduced the UVATS program. We felt that the stage-by-stage progression from simple to complex procedures was the safest way to proceed to allow the surgeons to gradually gain confidence and experience and master new skills without compromising patient safety.
In the following sections we describe the technical aspects and surgical steps to perform simple and complex segmental anatomical resections.
Technical aspects
Patient selection
Indications for UVATS segmentectomy include: (I) peripheral early-stage NSCLC smaller than 2 cm without nodal involvement; (II) ground glass opacities <2 cm in diameter; (III) deeply located metastases; (IV) benign lung nodules and management by wedge resection impossible and (V) patients with compromised lung function or poor performance status.
Anatomical landmarks and anatomical variations
A comprehensive knowledge of the anatomy, notably regarding the arterio-venous-bronchial pattern is mandatory. For this reason, a single-breath thin slice (1-mm) injected chest CT-scan is performed to identify anatomical variations and to obtain precise understanding of hilar broncho-vascular anatomy. The study of the CT-scan should be particularly relevant when planning complex segmentectomies due to anatomical variations in these segmentectomies. Even if the use of 3D reconstruction is highly recommended (16,17), it is not performed in our institution as we feel that the quality and resolution of standard injected CT scans are optimal. Additionally, we found that interpretation of the 3D reconstruction does not reflect the shape of the deflated lung, which is the surgical reality, thus may not necessarily be generalizable to, or even reflect complex clinical scenarios.
Nodule localization
Pre-operative localization of the pulmonary nodules is particularly valuable during UVATS in cases of non-visible or non-palpable tumors. First, it facilitates the identification of small and deeply located nodules and second, it helps to determine the ISP to achieve enough resection margin.
Many techniques are available to localize small and non-palpable lung lesions. These include CT-guided radiopaque devices (hook-wire and fiducials) (18), intraoperative ultrasonography (19), radioisotope CT fluoroscopy (20), electromagnetic navigation bronchoscopy (21), dyes (22), radionuclides (23), contrast medium injection (24), among others. So far, no prospective or randomized trials were published to compare these methods amongst themselves or to assess them versus the standard manual palpation. In our department, we use CT-guided hook-wire localization and report high success rates (98.3%). We find the dislodgment rate of 3.7% to be acceptable as the displacement is mild and does not impact surgical margins (25). Minor complications were observed with pneumothorax in 38%, but only 2.8% required chest tube insertion. No hemothorax or pulmonary air embolism were reported.
Resection margins
Data suggests that minimal recurrence rates can be obtained with segmentectomies, as well as survival rates on par with lobectomies for small-sized NSCLCs (<2 cm) if the surgical margins are wide enough (typically 2 cm) and pathologically negative (26). Our standard approach is to maintain a margin/tumor diameter ratio >1, in agreement with reports that high recurrence rate (85%) are observed when this ratio is <1 (27). This can be delicate in cases where a lesion crosses the ISP. In such cases, to guarantee the completeness of the segmental resection, we extend surgical margins beyond the traditional segmental limits, thus carry out an ‘extended segmentectomy’. In cases where the lesion spans two segments, we favor safety and recommend a combined segmentectomy or an extended wedge of the neighboring segment. This should ensure that surgical margins are wide enough. Sub-segmentectomy would be an alternative to standard wedge resection or combined segmentectomy, but reports are under investigation (28).
ISP
The identification of the ISP is one of the most challenging steps in UVATS segmentectomies. Classically, the ISP is defined by the intersegmental veins. Hence, a complete anatomic segmentectomy requires that the dissection take place following the boundary of the intersegmental veins. However, no anatomical landmarks are recognizable on the visceral pleura, thus the completion of the segmentectomy may be elusive. For complex segments, we use the systemic injection of indocyanine green (ICG) with near-infrared imaging-system (Novadaq Technologies; Toronto, ON, Canada). After division of the segmental vessels and bronchus, ICG is injected i.v. (2.5 mg/mL, 3 mL overall, peripherally). Then, the demarcation between the devascularized, ICG-enhanced tissues and the normal parenchyma is marked by electrocautery (29) (Figure 1). In our experience, 10% of patients have modified ISP anatomy, the safe resection of which avoids potential operative complications such as infarction or infection (14). Several authors have reported on the best way to delineate the ISP (inflation/deflation techniques) after the segmental bronchus closure (30,31). However, in our experience, the presence of collateral canals that enable retrograde inflation of the target segment despite the closure of the segmental bronchus, results in obstruction of the surgical view and makes the identification of the ISP difficult. This is particularly inconvenient in patients with emphysema if the lung does not deflate sufficiently during the operation. Recently, a simple technique described the use of collateral ventilation with insufflation of 100% of oxygen. About 15–20 minutes after single-lung ventilation, with the targeted segment inflated and the other segments collapsed, a defined line was developed that permitted to localize the ISP in 96.6% (32).
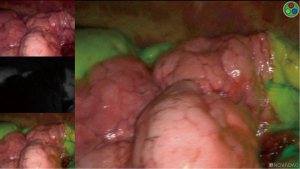
Peri-operative settings and instrumentation
The general anesthesia is managed with a left double-lumen tube. The patient is positioned on the side and the inter-costal distance is increased by flexing the table. A 3 to 5-cm incision is performed in the 4th or 5th intercostal space (for upper and lower segmentectomies, respectively) between the tip of the scapula and the breast in the anterior axillary line. The length of the incision may be adapted based on the patients’ size, thick thoracic wall, or obesity. The incision is retracted using a plastic soft tissue retractor (Alexis® Retractor Applied Medical USA), which also affords protection to the wound (Figure 2). The scrub nurse in charge is positioned posteriorly to the patient. The surgeon and assistant stand on the front side of the patient. This means that at least two monitors should be used. We strongly recommend the use of a 10-mm HD bent (30º) thoracoscope. This device will allow optimal visualization of all mediastinal and pleural structures.
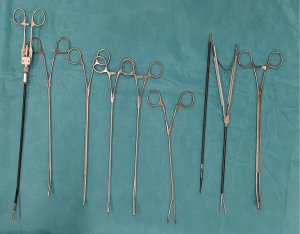
It is critical to obtain the right instruments to perform UVATS segmentectomies safely. For this reason, we use specific instruments with both proximal and distal articulation designed for uniportal procedures as follows: dissector, node grasper, Duval grasper, Foester clamp, Metzenbaum scissors, Debakey forceps, needle holder and knot pusher (Scanlan®, USA) (Figure 3).
We routinely use the monopolar hook cautery for dissection throughout surgery. We consider it to be a very precise instrument. However, caution should be exercised as the hook may be dangerous in untrained hands during vascular dissection. It is advisable to include ultrasonic shears or a bipolar sealing instrument within the armamentarium for hemostatic purposes. More specifically, we use the Ultracision Harmonic scalpel (Ethicon, Johnson & Johnson, USA), although any commercially available device is equally valid. A suction device should be active during the entire dissection maneuver for immediate evacuation of smoke and mist.
With regards to the division of the vessels and bronchus, we always use endoscopic staplers, meaning that all vessels, including the small segmental arteries are sectioned in this way. Some authors have reported safety with transection of small branches with bipolar devices, but we feel more comfortable with staplers. The curved-tip stapler may greatly facilitate the passage around the structures. The stapler must load the vessel without any force or friction, which implies a perfect vascular dissection. We avoid the use of clips because they may slip, conflict with staples or tear the vessel if they get caught on a swab.
A summary of the technical aspects and instrumentation for UVATS segmentectomy is shown in Table 1.
Table 1
| Anatomical landmarks and anatomical variations: thin slice injected chest CT-scan |
| Nodule localization: CT-guided hook-wire |
| Margin resection: margin/tumor ratio>1 |
| Intersegmental plane: systemic ICG injection with near-infrared imagery (Karl Storz®, Germany) |
| Peri-operative setting and instrumentation: 10-mm HD 30º scope, soft tissue retractor (Alexis® XS Retractor Applied Medical USA), specific instruments with both proximal and distal articulation designed for uniportal procedures (Scanlan®, USA), Ultracision Harmonic scalpel (Ethicon, Johnson & Johnson, USA), endoscopic staplers (Endo GIA, Medtronic, MN, USA) |
UVATS, uniportal video-assisted thoracoscopic surgery.
Surgical technique
Because of the limited access and angulation to operate by UVATS, we use single direction approach, meaning that targeted structures are transected in sequence from superficial to profound. Moreover, bimanual instrumentation is mandatory to achieve optimal exposure of the segmental structures and to avoid instrument malposition. For this reason, the camera is usually located in the posterior part of the incision and the instruments are placed below the camera.
We apply the following principles to conduct UVATS segmentectomies:
Exposure and lung retraction
Lung retraction and exposure of targeted structures is achieved by means of the suction device (the left hand is positioned in the posterior part of the incision), whereas the hook monopolar cautery is used to proceed with dissection (the right hand is positioned in the anterior part of the incision). Tension is adapted by pulling the suction more or less to facilitate entry into the dissection plane.
Mediastinal pleura, hilum, lung mobilization
Dissection starts by opening the pleura above the hilum posterior to the phrenic nerve with a hook monopolar cautery. The targeted lobe is pulled backward with the suction device to obtain adequate exposure. In lower lobe segmentectomies, the pulmonary ligament is incised up to the lower vein using both hook monopolar and gentle traction of the lower lobe anteriorly.
Division of the segmental artery
Arterial pattern should be completely clear by dissecting all segmental branches. It could be helpful to divide the fissure or part of the ISP first if the artery is deeply located in the fissure. After segmental arteries have been individually encircled, they are divided using an endoscopic vascular stapler. In case of segmental artery injury, compression is generally sufficient to achieve hemostasis due to the small caliber and low pressure of the vessels.
Dissection of the vein
The targeted vein is exposed and cleared from surrounding tissue. At this point, hilar lymph nodes are entirely dissected to facilitate identification of the vascular branching pattern. Avoiding mis-identification is key, and the best way to achieve it is to carefully dissect all venous branches on sufficient length. This might be delicate when venous anatomy is unusual or unclear. In such cases, we strongly advocate to divide the most obvious subsegmental vein and to continue opening the parenchyma above the vein with the hook monopolar cautery. Once the segmental vein is encircled, the lobe is retracted forward to place the stapler facilitated by a traction sling around the vein.
Interlobar, intersegmental lymph node clearance
Lymph node clearance is paramount during segmentectomy. First, it is a key-step to access the branches of the pulmonary artery. Second, it facilitates the exposure of the segmental bronchus. In this setting, it is compulsory to clear all the station 12 and 13 nodes before bronchial transection to avoid misjudgment. And third, it is a critical procedure to achieve optimal oncologic results.
Division of the segmental bronchus
Once the vein or artery has been divided (depending on the targeted segment), the vascular stump can be pulled towards the top using the suction device or a forceps to facilitate the exposure of the segmental bronchus. Again, further dissection into the parenchyma with hook monopolar cautery is usually necessary. At this point, we strongly encourage to remove all the station 12 and 13 lymph nodes around the bronchus before transection. Because of the small diameter and the narrow place around the segmental bronchus, it is looped with a silastic sling to facilitate the placement of the tip of the stapler.
ISP
To ensure a safe surgical margin of the tumor, the location of the tumor is marked by electrocautery dots. This is particularly important to avoid missing the tumor during ISP demarcation and conservation of normal lung parenchyma The ISP is identified through i.v. injection of ICG followed by near-infrared imagery after transection of the segmental arteries. The delineation between ICG-enhanced parenchyma and normal surrounding structures is clarified by electrocautery and the ISP is divided by successive applications of endoscopic staplers. It is highly recommended to always double-check the stapler jaws position by shifting camera location from the upper border to the lower border of the incision, to avoid hitting vascular structures. Because of that, the position of the camera is systematically shifted as follows: first, the camera is placed at the lower border of the incision to control the position of the thin jaw over the pulmonary artery. And second, the camera is placed at the top of the incision to check the position of the thick jaw of the stapler over the parenchyma.
Specimen retrieval and closure
The specimen is retrieved in a bag. Then, lymph node are systematically dissected. This is a generic procedure, common to all oncologic pulmonary resections. Thus, it is not described in this article. Finally, an intercostal block with local anesthesia (bupivacaine 0.5%) is applied between the 2nd and 8th intercostal space. A 28 French chest tube is placed, and the lung is re-expanded under direct vision. Post-operative course is generally managed by our enhanced recovery after surgery pathway (ERAS) (33).
Surgical tips and tricks for UVATS segmentectomy are shown in Table 2.
Table 2
| Suction device is essential for exposure and lung retraction. Tension and counter tension are regulated by pulling the suction device |
| Gentle traction of the targeted segment is a key trick to obtain adequate exposure and to properly identify the direction of the segmental bronchi and vessels |
| To complete the ISP, we strongly recommend to control broncho-vascular stumps and refer to them to follow the division of the parenchyma towards the segmental hilum. It is crucial to maintain the bronchial stump above the stapler in the targeted segment |
| The limited angulation inherent to UVATS may be overcome if the staplers are manipulated appropriately by keeping the angle cephalad |
UVATS, uniportal video-assisted thoracoscopic surgery.
Segmentectomies
Simple segments
Lower lobe apical segmentectomy (S6)
The interlobar pulmonary artery should be approached by first dissecting the major fissure. The superior segmental artery (A6) is transected with a stapler after full exposure of arterial branches. Then, the A6 stump is pulled upwards, and the apical segmental bronchus (B6) is dissected by means of hook monopolar cautery. At this point, opening of the posterior mediastinal pleura and performing subcarinal lymph node dissection can facilitate the exposure of B6. Once B6 is transected, the apical vein (V6) can be dissected by anterior or superior mobilization of the lower lobe and then transected with a stapler. Finally, the ISP is completed by means of successive applications of endoscopic staplers.
Lower lobe basilar segmentectomy (S7+8+9+10)
Dissection begins with the pulmonary ligament to mobilize the lower lobe and to expose the lower lobe vein. Then, the major fissure is opened and the interlobar pulmonary artery is identified. Once the anterior border of the artery is cleared from lymph nodes and surrounding tissue, the anterior aspect of the fissure is opened either by an energy device (in case of a thin fissure) or staplers (in case of a thick fissure). Thereafter, all the basilar arterial branches are dissected and divided. At this point, the lower lobe is retracted anteriorly and superiorly to divide the basilar branch of the lower lobe vein. Attention should be paid to preserve the superior tributary of the lower lobe vein (V6). Once the vein and the artery are cut, the basilar bronchus is fully exposed and divided. Basilar segmentectomy is completed by stapling the ISP. Careful inspection is mandatory to avoid injury of apical segment broncho-vascular structures.
Left upper tri-segmentectomy (S1+2+3)
Dissection starts by opening the mediastinal pleura. Then, attention is paid to dissect and divide the truncus anterior arterial branch. We prefer to first divide the segmental artery in order to facilitate the further venous division. At this point, the upper division of the superior pulmonary vein is exposed and dissected after identification of the lingular venous tributary. A silastic sling is used to loop the vein to minimize the tension when the vascular stapler is introduced. Dissection is carried out by gentle traction of the venous stump and by further dissection into the parenchyma with hook monopolar cautery to identify the left upper bronchus. The division of the upper segmental bronchus and the lingular bronchus is identified, and the tri-segmental bronchus is divided. Finally, the ISP is completed as previously described.
Lingulectomy (S4+5)
The major fissure is dissected to approach the branches of the pulmonary artery. Once the lingular artery is identified, the anterior aspect of the fissure is opened. Then, the superior vein is completely dissected and the lingular vein is divided. Thereafter, depending on the anatomical exposure, either the lingular artery or the lingular bronchus can be transected first. Lingulectomy is completed by stapling the ISP.
Complex segments
Upper lobe
S1apical segment
The anterior mediastinal pleura is opened. The apical segmental artery (A1) is exposed and severed after dissection of the truncus anterior pulmonary artery. Caution must be exercised to avoid transection of the anterior A3 in case of a common arterial trunk. Then, the arterial stump is gently pulled to dissect the bronchus that is hidden within the pulmonary parenchyma, and lymph nodes are totally removed. The upper bronchus is exposed peripherally to reveal B1, B2 and B3. The B1 is looped and transected with a curved tip vascular or parenchymal endostapler. The superior branch of the pulmonary vein is located deep in the posterior part of the central veins. V1 is identified by lung traction and divided. Finally, the ISP is stapled as previously described.
S2
First, the main fissure is opened to reveal the central vein and the pulmonary artery. A2, in its usual ascending course, is exposed and transected. Then, V2 that runs parallel to A2 is cut. The upper lobar bronchus is exposed peripherally to reveal B1, B2 and B3. At this point, attention is paid to avoid injury to the recurrent A2. After B2 is transected, the B2 stump is lifted and moved away from the hilum to dissect the parenchyma distally as much as possible to make room to place the stapler to complete the ISP.
S3
Dissection starts in the anterior mediastinum where the pleura above the hilum is approached. The superior vein is exposed and cleared from surrounding tissue. All branches must be carefully dissected on a sufficient length to avoid misidentification as follows: V1+2, V3 and V4+5. The V3 is encircled and transected. On the right side, the minor fissure is divided to facilitate exposure of the segmental artery and bronchus. The anterior segment is pulled towards the top to facilitate the exposure of the bronchus. B3 is identified by further dissection into the parenchyma with hook monopolar cautery and then transected with an endostapler. Thereafter, A3, that runs in the groove between V1+2 and the stump of B3 is encircled and cut. Then, the ISP is divided by successive application of endoscopic staplers.
Lower lobe
S8
The anterior part of the fissure is dissected, allowing exposure of the pulmonary artery. The basilar branches are dissected as distally as possible. A8 is dissected and cut by a vascular endostapler. Then, it is advisable to loop A9+10 for retraction to expose the anterior segmental bronchus underneath. By retracting B9+10 backwards, B8 is exposed. Care must be taken to go around B8 to avoid injury to V8 that lies behind. The segmental bronchus is transected. Thereafter, the bronchial stump is lifted up and V8 is dissected and severed. The ISP is divided along the demarcation line defined by the injection of indocyanine green (Video 1).
S9
The pulmonary artery is approached in the middle portion of the major fissure. The basilar arterial trunk is dissected. It is important to dissect all branches on a sufficient length to avoid misjudgment. Then, A9 is exposed, isolated and cut by a vascular endostapler. Once A9 is severed, the B9+10 bronchial branch can be identified and is located posterior to the A9 stump. The lateral segmental bronchus is isolated and cut. Care is taken to avoid injury to A10 that lies dorsal to B9. The V9 is then localized behind the bronchus and can be encircled and stapled. Then, the ISP is divided. This is the most difficult step as the ISP is tridimensional. It is strongly recommended to use i.v. ICG enhancement, with near-infrared imagery to visualize the demarcation line. It is important to include the broncho-vascular stumps in the specimen and to follow the division of the parenchyma in the direction of the segmental hilum (34).
S10
(I) Ligamentum-based approach
First, the pulmonary ligament is incised up to the lower vein using both hook monopolar and gentle traction of the lower lobe anteriorly. The inferior pulmonary vein is thoroughly dissected to expose its segmental branches. Care is taken to properly identify the superior branch (V6), that must be preserved. The posterior basal vein (V10) is identified and divided by vascular endostapler. The ISP between S10 and S7 is progressively opened by using an endostapler. This maneuver allows to safely continue further dissection of segmental bronchus and artery. Care is taken to insert the stapler above the intersegmental vein to avoid accidental transection of the remnant segmental veins. Then, the segmental bronchus B10 can be identified and divided after dissection of surrounding lymph nodes. Just behind it runs the segmental artery A10, which is encircled and cut by using vascular endostaplers. The ISP is finally separated from caudal to cranial direction (Video 2).
(II) Fissured-based approach
Dissection starts in the major fissure’s middle portion. This is where the artery is approached and allows clear identification of the common trunk for A9+10 and A6 (posteriorly). Then, dissection along the ISP proceeds between A9+10 and A6 until V6 is exposed. The V6 and the common basal vein are identified after pulmonary ligament incision. The dissection continues with the division of the ISP between S6 and S10 using an endoscopic stapler in order to separate the superior and basal segments. The pulmonary artery branches are easily recognized in the fissure and A10 can be easily identified and divided with an endoscopic vascular stapler. Then, posterior and cranial to the A10 stump, the B9+10 bronchial branch is easy to identify. After the division of B10, V10 is clearly identified. The (tridimensional) ISP division is a delicate step because of its topography. The ISP between S10 and S9 is divided by successive applications of endoscopic staplers. To this end, it is critical to include broncho-vascular stumps in the specimen and to follow the division of the parenchyma in the direction of the segmental hilum (35).
S9+10
(I) Ligamentum-based approach
First, the pulmonary ligament is incised up to the lower vein using both monopolar hook and gentle anterior traction of the lobe (Video 3). The inferior vein is dissected to expose its segmental branches. Care is taken to identify V6, which must be preserved. The inferior basal vein that drains V9 and V10 is cleared. V9 and V10 are divided separately. In cases of unclear anatomy, it is preferable to divide only the lower-most tributary. The ISP between S10 and S7 may be opened at this time to safely continue the dissection of segmental bronchi and arteries. The segmental bronchus B9+10 lies anterior to the vein stump. It is carefully dissected and encircled to guide the positioning of the stapler. Thereafter, the A9+10 branch is dissected and stapled using an endoscopic curved-tip vascular stapler. Finally, the ISP is completed, this being the most difficult step of the segmentectomy (36).
(II) Fissure-based approach
The pulmonary artery is approached in the major fissure. Interlobar lymph nodes are totally removed. All the arterial branches are exposed. Dissection proceeds incising the pulmonary ligament and opening the posterior mediastinal pleural. These structures are dissected to prepare the exit of the tip of the dissector when performing the intersegmental tunnel that will be created to separate S6 and S9+10. Then, dissection continues in the fissure. V6 is identified and dissection progresses between V6 and the inferior basal vein. A tunnel is created that connects the fissure with the posterior mediastinum and that separates S6 and S 9+10. In order to facilitate the insertion of the staplers, a silastic sling is used to pull the parenchyma. The tunnel is divided by endoscopic staplers. After the division of the ISP, the A9+10 become easily recognizable in the fissure, A9+10 is dissected and cut. Then, the bronchial branch B9+10 is isolated and cut. Thereafter, the inferior basal vein, that drains S9 and S10 is cut. Finally, the ISP is completed as previously described (37).
Comments
There is little doubt that segmentectomies represent a surgical challenge, especially when carried out using a UVATS approach. We believe that at the bottom of the learning curve, it might be beneficial to receive mentoring rather than learning solely from one’s own mistakes, which might precipitate premature abandonment of the technique. In addition, successfully adopting and mastering a new technique requires that one performs a certain number of procedures to gain confidence and make technical progress whilst avoiding any detriment to the patient.
Currently, segmentectomy is safe and feasible by UVATS. There is robust evidence that it is indicated in some early-stage NSCLC and metastases. We recommend adopting the single direction approach, that consists of sectioning the broncho-vascular structures in sequence (from superficial to profound) as it provides a simple and systematic method to succeed in simple and complex segmentectomies.
Acknowledgments
The authors wish to acknowledge the help of Matthieu Zellweger during manuscript preparation.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “VATS Segmentectomy”. The article has undergone external peer review.
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-28/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-28/coif). The series “VATS Segmentectomy” was commissioned by the editorial office without any funding or sponsorship. MG served as an unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). The manuscript is waived from patient informed consent according to the ethics committee or institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Asamura H, Okada M, Saji H, et al. Randomized Trial of Segmentectomy Compared to Lobectomy in Small-Sized Peripheral Non-Small Cell Lung Cancer (JCOG0802/WJOG4607L). In: 101st Annual Meeting of the American Association for Thoracic Surgery. 2021.
- Darras M, Ojanguren A, Forster C, et al. Short-term local control after VATS segmentectomy and lobectomy for solid NSCLC of less than 2 cm. Thorac Cancer 2021;12:453-61. [Crossref] [PubMed]
- Bennett WF, Smith RA. Segmental resection for bronchogenic carcinoma: a surgical alternative for the compromised patient. Ann Thorac Surg 1979;27:169-72. [Crossref] [PubMed]
- Miller JI, Hatcher CR Jr. Limited resection of bronchogenic carcinoma in the patient with marked impairment of pulmonary function. Ann Thorac Surg 1987;44:340-3. [Crossref] [PubMed]
- Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6; discussion 1667-8. [Crossref] [PubMed]
- Bédat B, Abdelnour-Berchtold E, Perneger T, et al. Comparison of postoperative complications between segmentectomy and lobectomy by video-assisted thoracic surgery: a multicenter study. J Cardiothorac Surg 2019;14:189. [Crossref] [PubMed]
- Gonzalez D, Delgado M, Paradela M, et al. Uni-incisional video-assisted thoracoscopic left lower lobectomy in a patient with an incomplete fissure. Innovations (Phila) 2011;6:45-7. [Crossref] [PubMed]
- Gonzalez-Rivas D. Single incision video-assisted thoracoscopic anatomic segmentectomy. Ann Cardiothorac Surg 2014;3:204-7. [PubMed]
- Bédat B, Abdelnour-Berchtold E, Krueger T, et al. Clinical outcome and risk factors for complications after pulmonary segmentectomy by video-assisted thoracoscopic surgery: results of an initial experience. J Thorac Dis 2018;10:5023-9. [Crossref] [PubMed]
- Ojanguren A, Gonzalez M. What is the optimal way to succeed in Uniportal VATS? J Thorac Dis 2020;12:3018-21. [Crossref] [PubMed]
- Yu PSY, Chan KW, Lau RWH, et al. Uniportal video-assisted thoracic surgery for major lung resection is associated with less immunochemokine disturbances than multiportal approach. Sci Rep 2021;11:10369. [Crossref] [PubMed]
- Handa Y, Tsutani Y, Mimae T, et al. Surgical Outcomes of Complex Versus Simple Segmentectomy for Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1032-9. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Mogi A, et al. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg 2018;66:81-90. [Crossref] [PubMed]
- Karenovics W, Gonzalez M. How to decrease technical obstacles to difficult video-assisted thoracoscopic surgery segmentectomy? J Thorac Dis 2019;11:53-6. [Crossref] [PubMed]
- Bédat B, Abdelnour-Berchtold E, Krueger T, et al. Impact of complex segmentectomies by video-assisted thoracic surgery on peri-operative outcomes. J Thorac Dis 2019;11:4109-18. [Crossref] [PubMed]
- Shimizu K, Nakazawa S, Nagashima T, et al. 3D-CT anatomy for VATS segmentectomy. J Vis Surg 2017;3:88. [Crossref] [PubMed]
- Chan EG, Landreneau JR, Schuchert MJ, et al. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2015;150:523-8. [Crossref] [PubMed]
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref] [PubMed]
- Wada H, Anayama T, Hirohashi K, et al. Thoracoscopic ultrasonography for localization of subcentimetre lung nodules. Eur J Cardiothorac Surg 2016;49:690-7. [Crossref] [PubMed]
- Doo KW, Yong HS, Kim HK, et al. Needlescopic resection of small and superficial pulmonary nodule after computed tomographic fluoroscopy-guided dual localization with radiotracer and hookwire. Ann Surg Oncol 2015;22:331-7. [Crossref] [PubMed]
- Luo K, Lin Y, Lin X, et al. Localization of peripheral pulmonary lesions to aid surgical resection: a novel approach for electromagnetic navigation bronchoscopic dye marking. Eur J Cardiothorac Surg 2017;52:516-21. [Crossref] [PubMed]
- Findik G, Demiröz SM, Apaydın SMK, et al. Computed Tomography-Guided Methylene Blue Labeling Prior to Thoracoscopic Resection of Small Deeply Placed Pulmonary Nodules. Do We Really Need Palpation? Thorac Cardiovasc Surg 2017;65:387-91. [Crossref] [PubMed]
- Galetta D, Bellomi M, Grana C, et al. Radio-Guided Localization and Resection of Small or Ill-Defined Pulmonary Lesions. Ann Thorac Surg 2015;100:1175-80. [Crossref] [PubMed]
- Mogi A, Yajima T, Tomizawa K, et al. Video-Assisted Thoracoscopic Surgery after Preoperative CT-Guided Lipiodol Marking of Small or Impalpable Pulmonary Nodules. Ann Thorac Cardiovasc Surg 2015;21:435-9. [Crossref] [PubMed]
- Hanauer M, Perentes JY, Krueger T, et al. Pre-operative localization of solitary pulmonary nodules with computed tomography-guided hook wire: report of 181 patients. J Cardiothorac Surg 2016;11:5. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32; discussion 932-3. [Crossref] [PubMed]
- Chang CC, Yen YT, Lin CY, et al. Single-port video-assisted thoracoscopic surgery subsegmentectomy: The learning curve and initial outcome. Asian J Surg 2020;43:625-32. [Crossref] [PubMed]
- Bédat B, Dackam S, Ojanguren A, et al. Near-infrared imaging for complex thoracoscopic resections. Video-assist Thorac Surg 2019;4:10. [Crossref]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Tsubota N. An improved method for distinguishing the intersegmental plane of the lung. Surg Today 2000;30:963-4. [Crossref] [PubMed]
- Yao F, Wu W, Zhu Q, et al. Thoracoscopic pulmonary segmentectomy with collateral ventilation method. Ann Thorac Surg 2021;112:1814-23. [Crossref] [PubMed]
- Forster C, Doucet V, Perentes JY, et al. Impact of Compliance With Components of an ERAS Pathway on the Outcomes of Anatomic VATS Pulmonary Resections. J Cardiothorac Vasc Anesth 2020;34:1858-66. [Crossref] [PubMed]
- Ojanguren A, Forster C, Gonzalez M. Uniportal VATS laterobasal segmentectomy (S9) of right lower lobe. Multimed Man Cardiothorac Surg 2020; [Crossref] [PubMed]
- Ojanguren A, Sauvain MO, Forster C, et al. Uniportal S10 segmentectomy by transfissural intersegmental tunneling. Video-assist Thorac Surg 2020;5:29. [Crossref]
- Ojanguren A, Sauvain MO, Gonzalez M. Uniportal ligamentum-based approach to posterolateral segments 9 and 10. Multimed Man Cardiothorac Surg 2020; [Crossref] [PubMed]
- Ojanguren A, Forster C, Gonzalez M. Uniportal VATS fissure-based approach to segments 9 and 10. Multimed Man Cardiothorac Surg 2020; [Crossref] [PubMed]
Cite this article as: Gonzalez M, Ojanguren A. How I do VATS segmentectomy: the uniportal approach. J Vis Surg 2022;8:22.



