Patient specific anatomy: the new area of anatomy based on computer science illustrated on liver
Introduction
Patient anatomy is the most important component of any surgical procedure definition. Modern anatomical description introduced by Andrée Vésale is based on a description of human anatomy from “human alive or having lived” represented by an average and standardized anatomy. All patients being different, the average anatomy has been defined by variation or exception. Since Andrée Vésale and his “De Humani Corporis Fabrica”, anatomy has been progressively improved thanks to new techniques and technologies, increasing variations but making the average anatomy more precise. This anatomy has a major benefit: it allows physicians to use standardized names and labels. Surgical procedures have then been more easily explained and described for improved knowledge sharing.
Over the past century, medical imaging has brought a new revolution: internal anatomy of a patient could be seen without any invasive technique. Current 3D and 4D medical imaging can thus provide today patient-specific anatomical data including geometry, topology and also function of organs. But this revolution has highlighted both main limits of the current anatomy. The first one is that interpretation of image information and of visible anatomical variations is totally dependent on the physician’s knowledge and can vary from one case to another. The second one is that variation description is ever more important, all patients being different. These drawbacks can sometimes be so great that they create mistakes in the anatomical description of patients and their associated surgical eligibility.
The liver is here a perfect illustration of such limits. Systemic chemotherapy of advanced colorectal cancer (CRC) produces a 9% 5-year survival rate with modern chemotherapy (1). On the opposite, surgery offers the foremost success rates against liver tumour (more than 50% 5-year survival rate). The 5-year survival rate exceeds 80% in case of liver transplant. Regretfully, less than 20% of patients are eligible to surgery due to anatomical limitation. Indeed, the eligibility is based on various criteria and rules such as the Milan criteria for liver transplants, or the 2006 San Francisco consensus rules for partial liver resection. This conference established that two adjacent liver segments can be separated with an adequate vascular inflow and outflow as well as biliary drainage and that the standardized Future Liver Remnant (FLR) (standardized FLR = remnant liver volume/liver volume) must be over 20% for patients with an otherwise normal liver, 30% for patients who have received extensive preoperative systemic chemotherapy, and 40% for patients with existing chronic liver diseases such as hepatitis, fibrosis or cirrhosis. Precise knowledge of the liver anatomy is thus a key point for any surgical procedure, including resection of liver tumours or living donor transplant, the surgical eligibility being linked to the definition of liver segments.
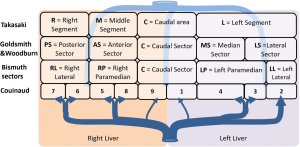
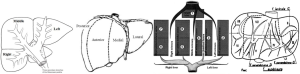
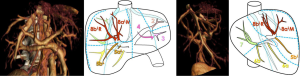
There are today four main anatomical definitions used in routine worldwide (Figure 1): the Takasaki segments definition (2) essentially used in Asia, the Goldsmith and Woodburne sectors (3) definition essentially in North America, the corrected Bismuth sectors (4) definition essentially used in Europe and the Couinaud segment (5) definition used worldwide.
These definitions are based on a labelling of the portal tree distribution in the liver following essentially geometrical criteria on relative location in the liver: right, middle, left, anterior, posterior, lateral, median and caudal. We can also notice that the hepatic veins define separating limits between main sectors in Goldsmith and Woodburne and Bismuth definitions. This general overview also clearly illustrates that Couinaud segmentation is the most precise one, all other segmentations can be obtained by a grouping of Couinaud segments in different sets. But Couinaud segmentation contains major errors. Platzer and Maurer (6) surely were the first ones to show in 1966 that the variability of segment contours was too important for any general scheme to be viable. Many research works (7-13) have subsequently completed that first study by providing quantifiable results thanks to 3D medical imaging. Couinaud himself (14) described in 2002 topographic anomalies. In 34 cases out of 111 (i.e., 30.63% of cases), he demonstrated that the real anatomical anterior sector of the liver (segment V + segment VIII) was different from his own definition. This may have surgical consequences. Thus, by clamping the right paramedian vein, portal branches which are topologically considered as being in segment VI took in fact their origin on the right paramedian branch, and were topologically in the anterior sector of the liver. Couinaud concluded that there was incoherence between vascular topology and the topography of the segments that could be corrected by using our 3D modelling and segmentation software (15) that we have clinically validated (16-19).
Indeed, the progress in imaging and computer sciences progressively allowed to visualize the portal and hepatic vascularization of the liver without pathology dissection. These works all showed that indirect landmarks, such as hepatic veins, are not suitable for a proper delineation of portal segments of the liver. Inappropriate delineation of the segments as defined by Couinaud classification can then lead to tumour localisation in an erroneous segment in about 16% of cases (study on 126 patients). Such an error should lead to reducing surgical eligibility. These various works illustrate and demonstrate the problem of modern anatomy based on an average patient and the necessity to develop a new personalized anatomy based on labelling and naming rules applied on 3D modelled medical images of the patient. We will present here such a new definition for liver surgery. In opposition with the Fasel definition (9) or other existing ones, this definition will be based on existing labelling (Takasaki, Goldsmith & Woodburne, Bismuth and Couinaud) that will be corrected by an easy labelling rule. It is thus easier to use in surgical routine.
Material and methods
For the following part of this article, we propose to extend the Bismuth comparison realized in 1982, in order to add Takasaki and Goldsmith and Woodburne descriptions of the liver segmentation. This general description clearly illustrates links and differences between the four main definitions (Figure 2). We will also replace the full name of segments or sectors by capital letters simplifying segment labelling.
In the current anatomical segmentations, when two branches (green and yellow arrows in Figure 3) of the portal network are pooled in a same segment or sector, and are thus labelled with a same label, four cases can arise:

- Both branches come from the same common portal branch and are drained by a same hepatic branch;
- Both branches come from the same common portal branch but are drained by two separate hepatic branches;
- Both branches come from two separate portal branches but are drained by a same hepatic branch;
- Both branches come from two separate portal branches and are drained by two separate hepatic branches.
Among these cases, only cases 1 and 2 allow to guarantee a correct topology in terms of labelling of portal branches. Indeed, a single ligature of the common portal branch is sufficient to stop the blood flow in this segment. This shows that applying a simple labelling rule would be enough to ensure correct topology for the labelling of portal branches. A new and unique “surgical” rule arises from this and can be defined as follow: two portal venous sub-networks can only be in a same segment, if and only if they come from the same crossing of a same portal branch. This purely topologic definition does not add any artificial topographic limitation so as to avoid the limitation or errors of existing segmentation. It allows to define segments of highly variable sizes according to the requested accuracy level. But this rule does not include any labelling mandatory to clinic description of tumour location. For the sake of rigour and in order to facilitate the use of that definition in clinical routine, we proposed to define a labelling from the four main label definitions described previously (see Figure 2). Correction of the label is done following the two new correcting rules:
- If a right (respectively a left) sector or segment is vascularized with a portal subtree coming from the left (respectively the right) portal vein, we add the letter L (respectively R) to indicate this unusual topological origin, which corrects standard surgical errors of the current segmentation. The same way, if a right or left sector or segment is vascularized with a portal subtree coming from the portal trunk, we add the letter T to indicate this unusual topological origin.
- When several subtrees with two different portal crossing origins vascularize a same area, we add a letter (a, b, c…) to differentiate their topological origin. Resulting segments have therefore different names in respect with our topological rule.
To these two labelling correcting rules, we have added two other rules, which are not mandatory to assume the topological rule but useful in practice to provide more detailed anatomical segmentation and thus more accurate surgical eligibility: - When several subtrees with a same portal crossing origins vascularize a same area, we can add a number (1, 2, 3…) to differentiate these different subtrees in a same segment. The labelling order, from 1 to N, is defined by following the clockwise direction from the portal crossing origin in an anterior view.
- When a segment is drained by only one left, median, right or accessory hepatic vein (case 1 of Figure 3), we can add a drainage letter L, M, R or A at the end of the new label.
The two correcting rules can be summarized by following letter addition:
+ L, R, T or M = left, right, tronc or middle portal branch origin;
+ a, b, c…if different venous origins for a same segment area.
The optional correcting rules can be summarized by following label addition:
+ 1, 2, 3…if a same venous origin for a same segment area;
+ R, M, L, A = right, median, left, accessory hepatic drainage
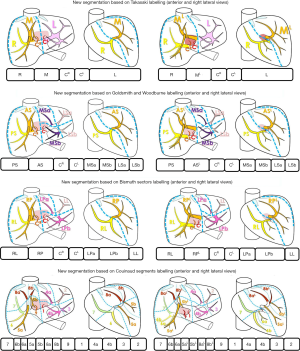
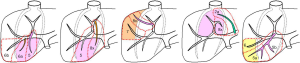
Applications of these correcting rules are illustrated on two different portal system distributions in Figure 4 from the four usual anatomical segmentation definitions. However, it is also possible to combine these different definitions. Indeed, the best way to proceed is to start from the most general one (Takasaki) to the most detailed one (Couinaud) according to the surgical need of precision. This need will be defined from the tumour location and from the vessels (portal and hepatic veins) that will define or complicate the surgical procedure. For instance, if no tumour is localized in the left liver, and if the median hepatic branch will not have to be resected by surgery, then it is not necessary to go over the Takasaki level of precision, a unique left segment is sufficient (Figure 5). If for the same clinical case a tumour is localized only in a part of segment 6 without any risk of sacrifice of the right hepatic branch, it will be possible to separate the right liver in a median segment, the right segment being separated by using the Couinaud level of precision and so associated labelling. In case of a tumour in segment 7 with a sacrifice of the right branch, the right liver will then be labelled following the Couinaud level of precision.
When applied, these rules provide a different anatomical segmentation even if close to the existing ones. What seems to be a small difference in the labelling provided by the addition of new letters is indeed significant as we will see in the result chapter. It is the main benefit of this new proposal, easy to apply because based on existing labelling used every day by all experts worldwide, but anatomically correct thanks to the corrective rules.
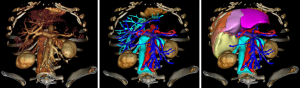
To be applied in clinical routine, this new definition requires the 3D visualisation of venous networks. A contrasted CT image at venous time (70 seconds after injection) or a MRI will have to be done so as to visualize these vessels thanks to direct volume rendering which is available on all current workstations. That volume rendering can also be obtained on a personal computer thanks to certified software such as OsirixMD (http://pixmeo.pixmeo.com/products.html#OsiriXMD) on Mac-OS, or free of charge VP-Planning (https://www.visiblepatient.com/en/products/software/) on Mac-OS and Windows. VP-Planning© visible patient integrates an automatic transfer function dedicated to vessel visualization. As illustrated in Figure 6, such volume rendering should be sufficient to define precisely the anatomical segment using our new definition.
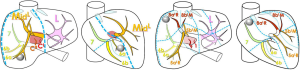
However, it is considers by physicians as complex to use. Another solution consists in using an image segmentation algorithm allowing to extract vessels from the medical image. To do it, several software tools are available on the market (Myrian© from Intrasense, Ziostation© from Ziosoft, Synapse© Vincent from Fujinon, Iqqa® Liver from Edda Technology, ScoutTM Liver from Pathfinder). Another solution consist in using distant 3D Modelling services (Mevis Distant Service, Visible Patient Service from Visible Patient) that do not request the purchase and use of expensive modelling workstations, the modelling being realized at distance by experts in image processing. Figure 7 illustrates the use of VP-Planning software after the Visible Patient Service has modelled a liver. As illustrated, the software allows for a virtual clip applying that provides in real-time the vascular territory of the clipped portal subtree defining the anatomical segment.
In order to clinically validate this new definition, a database of 20 injected CT images was set up. Images were acquired at venous time, i.e., 70 seconds after injection of the contrast medium. These images have been collected and anonymized after patient consent by the Digestive and Endocrine Surgery Department, University Hospital of Strasbourg, France. Patients have not been selected to be included in the database but their images. The single criterion was the quality of the CT image injected at venous time. Images of 10 women and 10 men, among which 2 women and 2 men had no hepatic pathology (i.e., 20%) have been collected. Women were aged between 38 and 62 and men were aged between 33 and 66. Five patients had a single tumour (25%), five patients had two tumours (25%), four patients had between three and eight tumours (20%) and two patients had more than 20 tumours (10%). This database presents a good variability of hepatic pathologies and features as many women as men.
A 3D modelling of the liver, its potential tumours and its hepatic and portal venous networks were provided by the Visible Patient Service. For each acquisition, an image in anterior and right lateral view has been edited. A hepatic surgeon was asked to delineate the standard Couinaud segmentation on each view. The same way, in parallel and blindly, computer scientists have indicated the computer-based segmentation on each view. In both cases segmentations have been realized with the 3D rendering software, allowing for a better vision of vessel localization. Finally, the results obtained by highlighting the most revealing differences were compared (Figure 8).
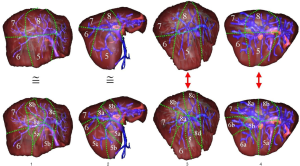
Results
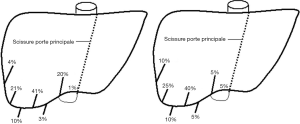
Out of the 20 images of the database, we note a revealing difference in 14 cases (70%) on at least one important branch of the portal network. Only six cases (30%) do not present a revealing difference between both labellings. The main differences summarized in Table 1 and illustrated on Figure 9 are as follows:

Full table
- In 11 cases (55%) a large branch, normally in segment V according to Couinaud’s segmentation, was topologically assessed to be located in one of segments VI according to the new definition. This figure even rose to 60% (12 cases) if smaller branches with that same labelling modification were integrated.
- In four cases (20%) a large branch, normally in segment V according to Couinaud’s segmentation, was topologically assessed to be located in one of segments VIII according to the new definition. This figure even rose to 30% (six cases) if smaller branches with that same labelling modification were integrated.
- In four cases (20%) a large branch, normally in segment VII according to Couinaud’s segmentation, was topologically assessed to be located in one of segments VIII according to the new definition. This figure doubles (40%) if smaller branches with that same labelling modification were integrated.
- In two cases (10%) a large branch, normally in segment VIII according to Couinaud’s segmentation, was topologically assessed to be located in segment IIa (called IVa in Couinaud’s classification) according to the new definition. These two atypical anatomies included a branch going from the left portal vein up to the cranial part of the liver such as a branch of segment IVa according to Couinaud, but reaching beyond the limit of the median hepatic vein to end up in the topographic territory of segment VIII according to Couinaud.
- In two cases (10%) a large branch, normally in segment VI according to Couinaud’s segmentation, was topologically assessed to be located in one of segments V or VIII according to the new definition.
Discussion
We propose herein a new anatomical segmentation of the liver aiming at correcting the topologic errors of Couinaud’s segmentation. To be applied, it requires a 3D visualization of portal and hepatic venous networks of the liver. The application of a simple labelling rule allows to guarantee a proper and logical anatomical segmentation. This first study carried out on 20 clinical cases showed a good correlation between its results and those observed in the literature. It moreover highlights the limits of Couinaud’s segmentation, which appears erroneous in more than 50% of cases when compared to our database for the definition of the segments of the right liver.
As expected, these results confirmed Couinaud’s observations reported in his recent study. But rather surprisingly, we found a revealing modification in segment V. Indeed, for over half of patients from the present database, at least one branch of segment VI according to the new definition was considered as belonging to segment V according to Couinaud’s classification. This particularity did not appear in the study published in 2002 and presenting a database of 111 cases. If such cases were present, they necessarily had to be part of the 77 cases (69.37%) sorted as being normal. In order to check the anatomic accuracy of our method regarding that difference of limit between segment V and VI, we proposed another method consisting in locating the right portal fissura (limit between segment V and VI) using the segment’s delineation. In the case of Couinaud’s anatomical segmentation, this fissure was theoretically located halfway between the right anterior angle and the main portal fissure. Couinaud indicated in his work (12) that this theoretical position was only found in 46 cases out of 111, i.e., 41.44% of cases. In fact, Couinaud indicated in that same work the real anatomical position of the fissure for the 111 cases, which is summarized on Figure 10. Thus, its position could be drawn in the same way in the new model of reconstruction, and it could be noted, as shown on Figure 6, that a good correlation between both results could be observed. This showed that the limit between segment V and VI provided by our new topologically corrected segmentation appears to correlate with the anatomic reality.
The present segmentation allows to achieve a segmentation similar to the sector segmentation described by Goldsmith & Woodburne, or a segmentation similar to that described by Bismuth using Couinaud’s classification (Figure 11). It defines a “true anatomical segment” based on a topologically correct labelling and merging of territories supplied by the portal venous sub-tree(s).
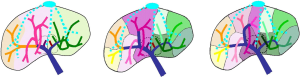
In comparison with other existing work, Fasel is the single author who has proposed to really modify Couinaud’s segmentation by proposing a new topologically correct definition called 1-2-20 in a recent work (14). The idea was to create a segment around each secondary branch originating from the left and right portal vein of the liver. By definition, this concept provided a topologically correct anatomy. However, by default it provided a very large number of segments in the left liver and rather few segments in the right liver. This was mainly due to the fact that in this work the left portal vein ended at the Rex Recessus, including thus the left paramedian vein while in the right liver the right portal vein was limited to the first main bifurcation. Moreover, variability of segment number resulting from the Fasel segmentation implied that a number did not represent an area. It was thus impossible to describe the location of a tumour by its number (the segment 6 for instance can be in the right paramedian or lateral sector, or in the left paramedian or lateral sector from one patient to another). Such a variability made its clinical application complex; all clinicians would have to use the same software.
Our presented study is limited to the evaluation of the right liver. It has to be completed by a similar analysis of the left liver which, according to Couinaud, should present fewer variations. However, the labelling of the branches of segment IVa according to Couinaud will at least entail a difference that has already been noted in the study of the right liver. Indeed, in two cases, we observed that a vein issued from the left portal branch joined the territory of segment VIII according to Couinaud. Renaming such branches into branch of segment IIa would illustrate a first variation which was featured in 10% of our cases. Further evaluation would consist in checking the potential clinical benefit provided by that anatomically corrected segmentation. A clinical study would have to allow the comparison of postoperative results of patients operated respecting Couinaud’s segmentation with patients operated following the new segmental definition of the anatomical segmentation. From a clinical point of view, this new segmentation process could allow to reduce tumour recurrence in patients operated for HepatoCellular Carcinoma (HCC), as it has been demonstrated that HCC has a portal segmental dissemination. It could further allow to reduce resected regions to smaller segments depending on tumour localisation.
Finally, it is furthermore interesting to note that this definition does not require any specific research or development on computer sciences level. In clinical routine, visualization through volume rendering will be sufficient to realize the presented labelling. Territories associated to each labelled branch can then be estimated on such 3D view knowing that direct volume rendering techniques are available on all current CT and MRI equipment as well as on certified software applications such as OSIRIXMD (on MacOS) or the free of charge Visible Patient Planning (on Windows and MacOs) (Figure 12).
Conclusions
We have proposed a new anatomical segmentation of the liver based on four main rules to apply in order to correct topological errors of the four main standard segmentations. Our validation clearly illustrates the large amount of mistakes created by the current standard definitions, increased by physician interpretation that can vary from one case to another. In the past, the only way to correct common anatomical mistakes was to clamp vessels during surgery, associated vascular territories appearing then clearly. By applying these rules, we can now obtain the same results preoperatively, these rules being based on the surgical logic of vascular territory clamping and using virtual reality technologies. Moreover, more recent software can simulate in the same way virtual clip applying on vessels and thus virtually provide the vascular territory in real-time. These rules should thus be applied in any organ to optimize and personalize their functional anatomical definition.
Acknowledgements
This work is a part of the 7th Framework Programme eHealth project PASSPORT, funded by the ICT priority of the European Community.
Footnote
Conflicts of Interest: Luc Soler and Jacques Marescaux have stock ownership of the Visible Patient company. The other authors have no conflicts of interest to declare.
Informed Consent:Written informed consent was obtained from the patient for publication. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol 2008;26:5721-7. [PubMed]
- Takasaki K.. Glissonean pedicle transection method for hepatic resection: a new concept of liver segmentation. J Hepatobiliary Pancreat Surg 1998;5:286-91. [PubMed]
- Goldsmith NA, Woodburne RT. The surgical anatomy pertaining to liver resection. Surg Gynecol Obstet 1957;105:310-8. [PubMed]
- Bismuth H.. Surgical anatomy and anatomical surgery of the liver. World J Surg 1982;6:3-9. [PubMed]
- Couinaud C.. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg 1999;16:459-67. [PubMed]
- Platzer W, Maurer H.. On the segmental arrangement of the liver. Acta Anat (Basel) 1966;63:8-31. [PubMed]
- Nelson RC, Chezmar JL, Sugarbaker PH, et al. Preoperative localization of focal liver lesions to specific liver segments: utility of CT during arterial portography. Radiology 1990;176:89-94. [PubMed]
- Soyer P, Roche A, Gad M, et al. Preoperative segmental localization of hepatic metastases: utility of three-dimensional CT during arterial portography. Radiology 1991;180:653-8. [PubMed]
- Fasel JH, Selle D, Evertsz CJ, et al. Segmental anatomy of the liver: poor correlation with CT. Radiology 1998;206:151-6. [PubMed]
- Rieker O, Mildenberger P, Hintze C, et al. Segmental anatomy of the liver in computed tomography: do we localize the lesion accurately? Rofo 2000;172:147-52. [PubMed]
- Fischer L, Cardenas C, Thorn M, et al. Limits of Couinaud's liver segment classification: a quantitative computer-based three-dimensional analysis. J Comput Assist Tomogr 2002;26:962-7. [PubMed]
- Strunk H, Stuckmann G, Textor J, et al. Limitations and pitfalls of Couinaud's segmentation of the liver in transaxial Imaging. Eur Radiol 2003;13:2472-82. [PubMed]
- Fasel JH. Portal venous territories within the human liver: an anatomical reappraisal. Anat Rec (Hoboken) 2008;291:636-42. [PubMed]
- Couinaud C.. Errors in the topographic diagnosis of liver diseases. Ann Chir 2002;127:418-30. [PubMed]
- Soler L, Delingette H, Malandain G, et al. Fully automatic anatomical, pathological, and functional segmentation from CT scans for hepatic surgery. Comput Aided Surg 2001;6:131-42. [PubMed]
- Bégin A, Martel G, Lapointe R, et al. Accuracy of preoperative automatic measurement of the liver volume by CT-scan combined to a 3D virtual surgical planning software (3DVSP). Surg Endosc 2014;28:3408-12. [PubMed]
- Soler L, Nicolau S, Pessaux P, et al. Real-time 3D image reconstruction guidance in liver resection surgery. Hepatobiliary Surg Nutr 2014;3:73-81. [PubMed]
- Mutter D, Soler L, Marescaux J.. Recent advances in liver imaging. Expert Rev Gastroenterol Hepatol 2010;4:613-21. [PubMed]
- Mutter D, Dallemagne B, Bailey Ch, et al. 3D virtual reality and selective vascular control for laparoscopic left hepatic lobectomy. Surg Endosc 2009;23:432-5. [PubMed]
Cite this article as: Soler L, Mutter D, Pessaux P, Marescaux J. Patient specific anatomy: the new area of anatomy based on computer science illustrated on liver. J Vis Surg 2015;1:21.