
Thoracoscopic S2 segmentectomy by a posterior approach for a central metastasis: a case report
Introduction
Central metastases are an ideal indication for pulmonary segmentectomy. However, the need for complete resection with negative margins requires attention to detail throughout the case, especially meticulous dissection at the root of the segment to create sufficient space for proper stapling of the parenchyma. A case of thoracoscopic S2 segmentectomy for a central metastasis in a 28-year-old man is presented. The case is presented in accordance with the CARE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-48/rc).
Case presentation
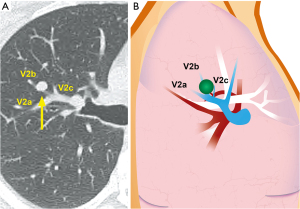
A 28-year-old patient with normal lung function was referred for a metastasis in the right upper lobe. The patient had undergone resection of a poorly differentiated sarcoma of the lower extremity 3 months prior, and had no other relevant medical history. Imaging showed a solitary, 5-Fluoro-Deoxy-Glucose-PET avid 9 mm nodule located centrally in the S2 bronchopulmonary segment (Figure 1). Given the typical findings, no preoperative histologic diagnosis was sought. The patient was taken to the operating room for a planned S2 segmentectomy. A simple wedge resection was not possible given the central location of the tumor.
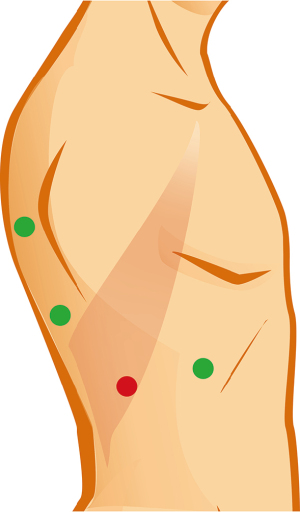
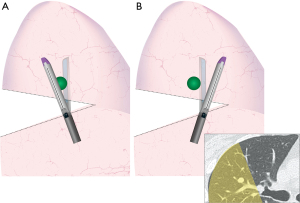
Patient positioning and surgical access are depicted in Video 1 and Figure 2. For S2 segmentectomies, the surgeon stands behind the patient and operates through two posterior ports. Although it is difficult to appreciate in the video (Video 1), in this particular case the low posterior trocar was positioned too low and the angle of attack to the hilum was too acute, resulting in a fulcrum effect at the chest wall compromising range of motion and haptic sensation. It made handling of the instruments tedious and caused recurrent oozing from the chest wall.
Dissection was begun by dividing the inferior pulmonary ligament, in order to give the lung full mobility and facilitate retraction and exposure of the posterior hilum (Video 1). The first key intraoperative view shows the angle at the bifurcation of the right upper lobe bronchus and bronchus intermedius (Figure 3A). Correct exposure requires precise and purposeful retraction of the lung along 3 axes: anterior, inferior, and counter clockwise rotation in relation to the hilum. These maneouvers are executed by the assistant using a grasper inserted through the anterior port (Video 1). Incorrect retraction is liable to « bunch-up » the lung and impede, rather than improve, exposure. The posterior pleural reflection is divided, and subpleural tissue is dissected to expose the angle at the bronchial bifurcation. Advanced bipolar cautery is ideal for this purpose, as it allows grasping, dissection, precise cauterisation with minimal heat diffusion, and tissue division, while limiting instrument substitution between steps.
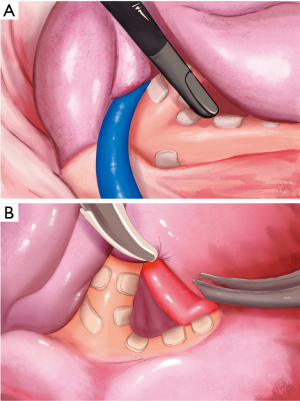
The second key view is the segmental artery A2 as it courses in the space above the bronchial bifurcation (Figure 3B). As this space is dissected and cleared of lymphatic tissue, the artery comes into view. It is important to correctly identify the A2 branch to the upper lobe, as in this case, A2 and A6 shared a common origin. Dissection of the bronchial bifurcation and A2 are the most important and potentially difficult steps in the operation. Once A2 is identified, dissection can be carried forward along the artery, underneath the parenchyma. As the fissure is divided (Figure 4), exposure is gradually improved, and access to the structures at the root of the segment, beginning with the artery, becomes straightforward.
Dividing the artery leads to the third key view consisting of the segmental bronchus B2 and venous trunk V2 (a + b + c) (Figure 5A,5B). B2 is dissected carefully while hugging the bronchus, being mindful of V2 coursing just behind. B2 is then divided.
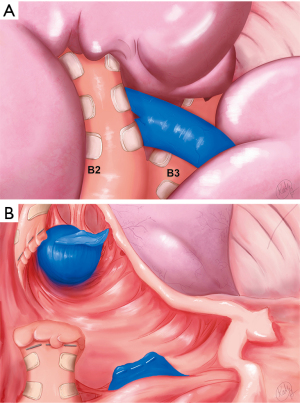
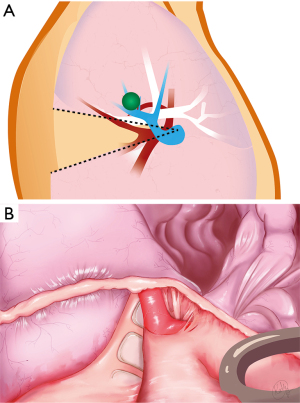
Dividing the vein is the key step that allows the space at the base of the segment to open up. This is especially important when resecting central lesions, as one requires sufficient space to position the anvil of the stapler properly to insure adequate margins (Figure 6). The inflation/deflation line is the author’s preferred way of delineating the intersegmental plane (not shown). The intersegmental plane is divided. A recurrent A2 artery (shown schematically in Figure 1) is included en bloc in the parenchymal staple line. The specimen is removed in a retrieval bag. Frozen section examination confirmed complete resection with negative margins.
The author uses fibrin-sealant (Flo-Seal®) on all staple lines because the literature suggests some benefit on the incidence and duration of air leaks (1), while recognizing that the efficacy of such an approach is not definitively established.
Operative time was 150 minutes, and blood loss was 300cc. The patient did well postoperatively. The chest tube was removed on postoperative day 1, and he was discharged on postoperative day 3. Final pathology confirmed the diagnosis of a 12-mm metastatic sarcoma. The parenchymal and bronchial margins were 20 and 10 mm, respectively. After review of the case by a multidisciplinary soft tissue oncology team, no systemic therapy was administered (either before or after the surgery). Imaging remains negative at 4 months.
The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying video. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Anatomic sublobar resection allows preservation of parenchyma and is particularly well-suited to patients who are old, frail, or whose physiology is otherwise compromised (2). It is increasingly considered for the resection of early-stage and low-grade lung cancer, although it remains somewhat controversial in this setting (3-5). Given the different clinical behaviour of metastatic tumors which normally require only resection with negative margins, central metastases are an ideal indication for anatomic sublobar resection. Although it is true that what constitutes adequate margins in this context remains the subject of debate, practice at the author’s institution as to the extent of resection and eligibility for a segmentectomy includes a detailed risk-benefit assessment of each individual case, including the patient’s clinical parameters and tumor histology/biology. From a purely technical perspective, it has been our experience that pathologically negative margins are achievable using the technique described, even for lesions located centrally in the segment. Furthermore, segmentectomy allows, adequate, safe resection, with preservation of lung function, decreased morbidity, and decreased length of stay (6,7). Parenchymal sparing also allows for more flexibility in the event of future metastatic disease requiring treatment (7).
Although several approaches have been described, the author finds that a direct posterior approach to S2 minimizes dissection and allows sparing of the horizontal fissure, reducing the potential for prolonged air leak. As such, it also may be particularly useful in the case of a completely fused fissure. Sparing tissue planes will also facilitate redo-surgery (whether wedge, segmentectomy, or lobectomy) and increase therapeutic options in the event of future metastatic disease (8). Although segmental venous anatomy can be properly delineated by an anterior approach, a careful posterior approach can also allow one to properly identify segmental venous branches (see Video 1).
Anatomic sublobar resection is a game of details, and each step will determine whether the operation runs smoothly or runs into speedbumps. Correct positioning of trocars and purposeful retraction are key. Although “3 trocar” approaches have been described, the author currently favours a “4 trocar” approach for ease of retraction, manipulation, and versatility in case of unexpected findings. When inserting trocars, it is essential to obtain an optimal angle of attack to the operative target, which will insure comfortable and safe dissection. This will also minimize fulcrum effects which may otherwise result in local trauma to the chest wall, increased bleeding, and intercostal nerve injury. Incorrect positioning of the low posterior trocar is a technical error that, although surmountable, definitely increased the level of difficulty in the case described (9).
Because of the angle of the camera and instruments, the most difficult portion of the dissection is identification of the artery in the bronchial angle. The author’s standard approach is to identify the artery first, before dividing the posterior portion of the fissure (between S2 and S6). Alternatively, to facilitate initial exposure, a portion of the fissure may be divided before the artery is identified. However, this risk injuring the artery or unwittingly including a portion of the artery in the staple line, making it vulnerable to avulsion. In the author’s experience, there was one case where dividing the posterior fissure before sufficient dissection resulted in staples landing very close to the A2 artery, making subsequent dissection of the artery extremely delicate. With proper positioning of the trocars, deliberate retraction and a bit of patience, the author has found that developing a plane above the artery before introducing the stapler is quite feasible. Dividing the inferior pulmonary ligament is an important manoeuver that gives the lung full mobility and allows the assistant to rotate the lung counter clockwise which greatly helps exposure during this portion of the surgery.
Advanced imaging including 3D reconstruction and 3D printing is increasingly being considered in sublobar resection surgery for operative planning and even intraoperative guidance (10,11). As more and more challenging cases are being considered, advanced imaging will become an essential part of the surgeon’s toolkit. It will allow determination of exact bronchovascular anatomy and any anatomic variations, as well as the precise relationship between the tumor, bronchovascular structures, and intersegmental plane, allowing for the anticipation of any technical difficulties while reducing the cognitive load on the surgeon. Since routine access to advanced 3D reconstructions is still not available at the author’s institution, operative planning and guidance must rely on standard Chest-CT, which is possible but represents an additional challenge, requiring some familiarity and experience.
Finally, a challenge specific to the resection of central lesions is the requirement for sufficient space at the base of the segment to allow proper positioning of the stapler. Otherwise, one risks positive margins. This requires methodical management of the segmental vein V2 (12). In the case presented, the lesion was extremely close to the bifurcation of veins V2a, b and c (Figure 1), which could therefore not be preserved. Cross-sectional imaging documented proximal collateral venous drainage from B1 and B3, which would be unaffected by division of the V2 venous trunk. Dividing the V2 (a + b + c) trunk as shown in this case allows the space at the base of the segmental hilum to open up, and allows one to carry the dissection plane at the root of the segment forward as far and wide as possible (Figure 5).
The author finds that stapling, as opposed to dissection of the intersegmental plane, allows one to take full advantage of this space at the base of the segment. Preoperative imaging showed that the nodule in the case described was located close to the B1-B3 intersegmental plane and optimal dissection and positioning of the stapler were necessary to allow the surgeon to encroach just enough on adjacent parenchyma beyond the intersegmental plane to ensure an oncologically sound resection (Figure 6). Although division of the V2 (a + b + c) trunk may in theory affect a portion of the venous drainage of S1 and S3, the author has used this technique in 11 cases of S2 segmentectomy [10 have been published previously as part of a larger series (13)] without any adverse postoperative issues: the median duration of chest tube drainage was 1 day (range, 1–3; IQR: 1–1.5) and the median LOS was 2 (range, 1–5; IQR: 1–3.5); there were no complications in this group of patients.
Conclusions
Central metastases are an ideal indication for segmentectomy, and the author has described the technique for a posterior thoracoscopic approach to S2. While different approaches to S2 have been described, it is important to recognize that while there is not one single possible approach, a thorough understanding of individual approaches will allow surgical teams the versatility to tackle a variety of specific surgical challenges.
Acknowledgments
The author wishes to thank the audio-visual department of Maisonneuve-Rosemont Hospital, in particular Sylvain Durocher, Mathieu Favreau and Kathy Hernandez, for contributing their talent, for their professionalism, and for their ongoing support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michel Gonzalez) for the series “VATS Segmentectomy” published in Journal of Visualized Surgery. The article has undergone external peer review.
Reporting Checklist: The author has completed the CARE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-48/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-48/coif). The series “VATS Segmentectomy” was commissioned by the editorial office without any funding or sponsorship. GR has received speaker fees from Medtronic® and Astra-Zeneca® and will be receiving research funding from Baxter®. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McGuire AL, Yee J. Clinical outcomes of polymeric sealant use in pulmonary resection: a systematic review and meta-analysis of randomized controlled trials. J Thorac Dis 2018;10:S3728-39. [Crossref] [PubMed]
- Cheng K, Zheng B, Zhang S, et al. Feasibility and learning curve of uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2016;8:S229-34. [PubMed]
- Zhang Y, Liu S, Han Y, et al. Robotic Anatomical Segmentectomy: An Analysis of the Learning Curve. Ann Thorac Surg 2019;107:1515-22. [Crossref] [PubMed]
- Duan L, Jiang G, Yang Y. One hundred and fifty-six cases of anatomical pulmonary segmentectomy by uniportal video-assisted thoracic surgery: a 2-year learning experience. Eur J Cardiothorac Surg 2018;54:677-82. [Crossref] [PubMed]
- Chang CC, Yen YT, Lin CY, et al. Single-port video-assisted thoracoscopic surgery subsegmentectomy: The learning curve and initial outcome. Asian J Surg 2020;43:625-32. [Crossref] [PubMed]
- Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. [Crossref] [PubMed]
- Wu W, Xu J, Wen W, et al. Learning curve of totally thoracoscopic pulmonary segmentectomy. Front Med 2018;12:586-92. [Crossref] [PubMed]
- Rakovich G. Thoracoscopic apicoposterior segmentectomy, left upper lobe: Posterior approach. Multimed Man Cardiothorac Surg 2021; [PubMed]
- Raveglia F, Cioffi U, De Simone M, et al. Advantages of wound retractor device versus rigid trocar at camera port in video-assisted thoracic surgery-a single institution experience. J Vis Surg 2018;4:66. [Crossref] [PubMed]
- Hernandez-Arenas LA, Purmessur RD, Gonzalez-Rivas D. Uniportal video-assisted thoracoscopic segmentectomy. J Thorac Dis 2018;10:S1205-14. [Crossref] [PubMed]
- Seguin-Givelet A, Grigoroiu M, Brian E, et al. Planning and marking for thoracoscopic anatomical segmentectomies. J Thorac Dis 2018;10:S1187-94. [Crossref] [PubMed]
- Nomori H, Okada M. Illustrated anatomical segmentectomy for lung cancer. 1st ed. Springer Science & Business Media, 2011:35-41.
- Rakovich G, Belahmira G, Woodall WH, et al. Learning curve for completely thoracoscopic anatomic sublobar resection. Minerva Surg 2022;77:101-8. [Crossref] [PubMed]
Cite this article as: Rakovich G. Thoracoscopic S2 segmentectomy by a posterior approach for a central metastasis: a case report. J Vis Surg 2022;8:39.


