Single stage robotic total mesorectal excision—a stepwise approach
Introduction
Development of laparoscopic surgery over the past two decades has given a new paradigm in management of colorectal cancers. Various randomized controlled trials have demonstrated its short-term and long-term benefits over conventional open surgery in the treatment of colon cancer such as faster recovery, decreased morbidity and reduced hospital length of stay with comparable oncological result and survival outcome (1,2). However, laparoscopic surgery has some limitations such as 2-dimension view, unstable assistant controlled camera, poor ergonomics, straight tip instruments, fulcrum effect and enhanced tremor effect.
To overcome these limitations, robotic surgery is a new technique with the benefits of a three-dimensional view, the ability to use multi-degree-of-freedom forceps, the elimination of physiological tremors, and a stable camera view. Performing surgery in a narrow pelvic cavity with conventional laparoscopy is a challenging procedure, especially in patients undergoing rectal cancer surgery. Robotic surgery is a new modality to overcome these difficulties (3-6). Several studies have demonstrated the safety and feasibility, as well as acceptable short-term outcomes, of robotic colorectal surgery (7-10).
This video article will describe a stepwise approach of single stage robotic total mesorectal excision (TME).
Clinical summary
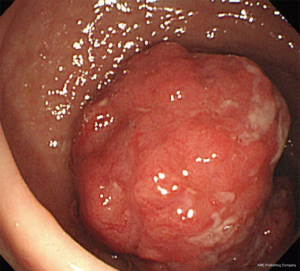
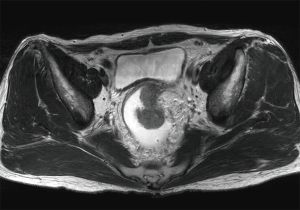
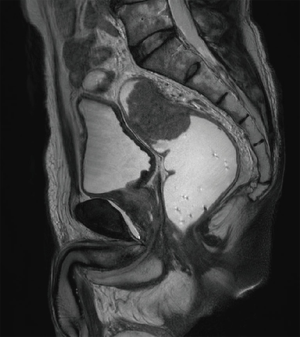
A 56-year-old man presented with a bowel habit change. Digital rectal examination (DRE) shows rectal mass located 8 cm from anal verge (AV). Colonoscopy revealed a malignant looking mid rectal mass, no others synchronous lesion was found (Figure 1). The biopsy shows moderately differentiated adenocarcinoma. Clinical staging with magnetic resonance imaging (MRI) show mid-rectal tumor with clear circumferential margin with several lymph nodes (Figures 2,3). Chest and abdominal computed tomography (CT) show no distance metastasis. Patient was staged as cT3N2M0.
Patient selection and workup
Robotic TME was selected for unfavorable tumor characteristic patients such as mid and low rectal cancer, male and obese patients. Colonoscopy was routinely performed to confirm histological diagnosis on biopsy and to role out other synchronous lesions. The distance between the tumor and AV was assessed via DRE and/or rigid sigmoidoscopy. Patients were staged according to the American Joint Committee on Cancer staging manual (7th edition). Clinical staging was performed via pelvic MRI, and a whole abdominal and chest CT.
Pre-operative preparation
Preoperative preparation begins with a full history and physical examination. Blood tests should be including a full blood count, electrolytes, liver function tests, carcinoembryonic antigen (CEA) level. An electrocardiogram is requested, where appropriate, cardiopulmonary exercise testing is done to stratify operative risk and optimize perioperative management. In our institution, a mechanical bowel preparation is not routinely given, otherwise, patient has only one time rectal enema a day before surgery. Fasting is maintained for at least 6 hours before surgery. Prophylaxis antibiotic was given 30 minutes before surgery.
Operating room setup, patient positioning, and ports placement
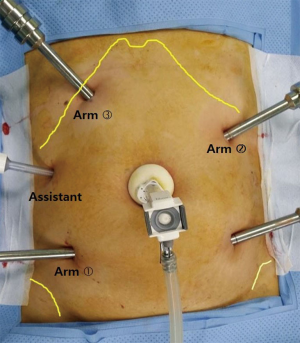
After the induction of general anesthesia, patient is placed in a modified lithotomy position with the legs apart on a bean’s bag mattress to prevent any sliding. Six ports are used (Figure 4), including one 12-mm camera port, four 8-mm robotic working ports, and one 5-mm port for assistant. A 12-mm trocar is placed through a peri-umbilical incision for the robotic camera. The intraabdominal pressure is maintained at 8–10 mmHg. The first daVinci® 8-mm port on right lower quadrant (RLQ) is placed at the Mc Burney point. The second port on right upper quadrant (RUQ) is inserted in the right subcostal area on the midclavicular line (MCL). The third port is placed in the left upper quadrant (LUQ) approximately 1–2 cm above the camera port at the crossing of MCL. The fourth port is inserted in the left lower quadrant (LLQ) approximately 1–2 cm lateral to the MCL. These four ports are used for the robotic arms and are separated from each other by at least 8 cm. To allow the assistant access, a 5-mm trocar is placed in the right flank area, near the anterior axillary line, at the umbilical level. This is used for suction/irrigation, clipping of vessel, and retracting of tissues. During pelvic dissection stage, the assistant uses the RUQ port as well, therefore maximizing assistance by use of both hands.
Procedure
Our technique is called a single-stage procedure because the surgery is performed without changing the position of the robotic cart. The surgical procedure (Figure 5) was divided into three phases (12-14).

Abdominal phase: vascular ligation, and sigmoid colon to splenic flexure mobilization
The patient is tilt to the right side and placed in the Trendelenburg position. The small bowel loops retracted away from the pelvic cavity to the RUQ to expose the inferior mesenteric artery (IMA). Before docking of the robot, the whole abdominal cavity is explored by conventional laparoscopic instruments. The robot cart is positioned obliquely at the LLQ along the imaginary line from the camera port to the anterior superior iliac spine (Figure 6). Then, the robotic arms are docked to the trocars. A zero degree robotic camera is used. A monopolar curved scissors is used by the RLQ arm as the surgeon’s right hand. A Maryland bipolar grasper forceps is taken by the RUQ arm as the surgeon’s left hand, and a Cardiere grasper are used by the LUQ arm as the surgeon’s second left hand. In this phase, the LLQ port is not used (Figure 7). Initially, the mesocolon over the IMA is retracted upwardly with a Cardiere forceps. The peritoneum around the base of IMA is incised and dissected with a monopolar scissors. The periaortic hypogastric nerve plexus is carefully preserved. The IMA is divided near the root (high ligation) with Hem-o-lok® clip. The inferior mesenteric vein (IMV) is identified by dissecting superiorly toward the ligament of Treitz, and is divided near the inferior border of pancreas. The medial dissection continues laterally until the left colon is separated from the retroperitoneum, and superiorly over the pancreas until the lesser sack is entered. The left gonadal vessels and ureter are identified and preserved. Lateral detachment is initiated along the white line while the sigmoid colon retracted medially by the assistant. The lateral counter traction by the LUQ arm will facilitate a safe dissection. Lateral dissection contininues cephalad from the proximal part of the decending colon to splenic flexure.
Pelvic dissection phase
The robotic instrument of the RUQ and LUQ ports are dedocked to the LUQ and LLQ ports, respectively (LUQ for Maryland forceps and LLQ for Cardiere forceps). Now assistant uses the RUQ port for cephalad traction of sigmoid colon and 5 mm port for suction. Therefore, five instruments are used (Figure 8) in the operative field (three robotic and two handheld), maximizing assistance for TME. An avascular plane between the mesorectal fascia and the presacral fascia is shaply dissected with a monopolar scissors. The inferior hypogastric nerves and distally, the pelvic nerve plexus are identified and preserved. Because the small bowel would obscure the right lateral plane, further posterior dissection down to the levator ani muscle is approached from the left lateral plane, while the rectum lifted up by the Cardiere graspers. The left lateral dissection was performed while the rectum was drawing to the right side by assistant. The right lateral dissection is completed in a reverse fashion of rectal retraction. Finally, the anterior dissection was performed by incising the peritoneal reflection. Shap dissection continues to develop the correct plane between the rectum and the seminal vesicle-prostate/vaginal. Pelvic dissection was performed to the level of pelvic floor muscles.
Rectal reconstruction with or without ileostomy
The rectum and sigmoid colon are delivered via RLQ port site for further ileostomy site. Sometimes if the tumor is too big or the mesentery is too bulky, the specimen is delivered via a mini-laparotomy incision on the LLQ port. After transection of the specimen, reconstruction of bowel continuity was performed using end to end anastomosis with CDH 29. After anastomosis was done, air leak test was performed. Finally a closed suction drain was inserted in pelvic cavity.
Post-operative management
- Begin clear fluid intake as soon as possible;
- Start soft diet on post-operative day 3;
- Remove Foley catheter on post-operative day 4;
- Drainage catheter removal on post-operative day 5;
- Discharge on post-operative day 10.
Tips, tricks and pitfalls
- To prevent hypogastric nerves injury, IMA should be clipped at 1 cm distal to the IMA-aorta junction;
- The high ligation of IMA and IMV, complete splenic flexure mobilization, and medial peritoneal detachment from pancreas are the keys to provide adequate colon length for tension free anastomosis;
- Complete pancreas separation from mesocolon is an important step to prevent pancreatic tail injury during splenic flexure mobilization;
- During medial to lateral dissection of the sigmoid colon, preservation of left ureter and gonadal vessel must be the first priority;
- Injury of marginal artery may occur during detachment gastrocolic ligament from transverse colon;
- Use a nylon tape for cephalad traction of upper rectum to facilitate good exposure and to get a proper TME plane;
- Distal resection margin 2–3 cm from tumor is the goal;
- Before apply stapler for rectal transection, clearing the mesorectal fat at the level of transection line should be done;
Conclusions
Single stage robotic TME is feasible with acceptable outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethnical committee. Written informed consent was obtained from the patient. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial.
- Colon Cancer Laparoscopic or Open Resection Study Group, Buunen M, Veldkamp R, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol 2009;10:44-52. [PubMed]
- Baek SJ, Kim CH, Cho MS, et al. Robotic surgery for rectal cancer can overcome difficulties associated with pelvic anatomy. Surg Endosc 2015;29:1419-24. [PubMed]
- Park S, Kim NK. The Role of Robotic Surgery for Rectal Cancer: Overcoming Technical Challenges in Laparoscopic Surgery by Advanced Techniques. J Korean Med Sci 2015;30:837-46. [PubMed]
- Ramos JR, Parra-Davila E. Four-arm single docking full robotic surgery for low rectal cancer: technique standardization. Rev Col Bras Cir 2014;41:216-23. [PubMed]
- Mak TW, Lee JF, Futaba K, et al. Robotic surgery for rectal cancer: A systematic review of current practice. World J Gastrointest Oncol 2014;6:184-93. [PubMed]
- Delaney CP, Lynch AC, Senagore AJ, et al. Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum 2003;46:1633-9. [PubMed]
- D’Annibale A, Morpurgo E, Fiscon V, et al. Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum 2004;47:2162-8. [PubMed]
- Woeste G, Bechstein WO, Wullstein C. Does telerobotic assistance improve laparoscopic colorectal surgery? Int J Colorectal Dis 2005;20:253-7. [PubMed]
- Choi DJ, Kim SH, Lee PJ, et al. Single-stage totally robotic dissection for rectal cancer surgery: technique and short-term outcome in 50 consecutive patients. Dis Colon Rectum 2009;52:1824-30. [PubMed]
- Priatno E, Kim SH. Robotic TME: a stepwise approach. Asvide 2015;2:157. Available online: http://www.asvide.com/articles/734
- Kim SH, Shin JW. Robot-assisted intersphincteric resection. In: Schiessel R, Metzger P, editors. Intersphincteric Resection for Low Rectal Tumors. New York: Springer-Verlag Wien, 2012:159-63. Available online: http://www.springer.com/cn/book/9783709109281
- Kim SH, Kwak JM. Robotic total mesorectal excision: operative technique and review of the literature. Tech Coloproctol 2013;17 Suppl 1:S47-53. [PubMed]
- Hellan M, Stein H, Pigazzi A. Totally robotic low anterior resection with total mesorectal excision and splenic flexure mobilization. Surg Endosc 2009;23:447-51. [PubMed]
Cite this article as: Priatno E, Kim SH. Single stage robotic total mesorectal excision—a stepwise approach. J Vis Surg 2015;1:24.