Minimally invasive repair of fenestrated atrial septal aneurysm
Introduction
Atrial septal aneurysm (ASA) is an uncommon congenital anomaly with a reported prevalence of 1). Anatomically, it consists of a redundant, bulging atrial septum. Although there is variation in the definition, generally accepted criteria include a basal width of at least 15 mm and amplitude of excursion of at least 10 mm from atrial septal plane (2). An interatrial communication is reported in 54% of patients with ASA (3). The clinical importance of this entity is its association with cryptogenic stroke (4), reported in 20–52% of patients with known ASA (5-7). The risk for recurrent stroke in patients with ASA with patent foramen ovale is reportedly 15% (8).
Medical, percutaneous and surgical treatments have been employed (9), but the superior modality remains unproven due to wide variation in study designs and definitions used and the lack of randomized trials. Some analyses have suggested the possible superiority of closure of the interatrial shunt over medical therapy (10); however, percutaneous closure is still associated with rare recurrent embolic events (11). The safety of surgical management has been described (12) and its superiority over medical therapy has been suggested by meta-analysis (13). The traditional surgical approach to ASAs has been median sternotomy which is highly invasive and is associated with a substantial recovery period. With modern advances in surgical visualization, minimally invasive techniques and peripheral cannulation for cardiopulmonary bypass, a right anterior minithoracotomy may be now used.
We describe the case of an otherwise active and healthy 47-year-old male who presented with a retinal embolic event who was found to have a large ASA and no other identifiable etiology on thorough investigation. Endovascular septal occlusion could not be performed due to the excessive, mobile septum and extensive fenestrations that would preclude complete elimination of interatrial shunting. Therefore, a minimally invasive surgical repair was offered.
Patients and methods
Patient selection and workup
Diagnosis of an ASA is made on the basis of TEE, since transthoracic echocardiogram may not detect TEE-proven ASA in up to 47% of cases (3). In modern practice, patients selected to undergo surgical repair of an atrial septal defect include those with cryptogenic stroke or complications of right ventricular volume overload who are ineligible for percutaneous closure. Most surgical patients would be considered for minimally invasive repair unless contraindicated by previous right thoracic surgery with significant adhesions, poor pulmonary reserve that would preclude the use of single lung ventilation or inadequate vascular access for peripheral cannulation.
Pre-operative preparation
All patients with risk factors for coronary atherosclerosis are evaluated with coronary angiography. Right heart catheterization may be helpful in defining the degree of pulmonary overcirculation or hypertension for larger left-to-right shunts identified by echocardiography. Patients with significant pulmonary pathology undergo pulmonary function testing and preoperative optimization.
Equipment preference card
Standard instrumentation for open cardiac and peripheral vascular surgery should be available. Additionally, many specialty instruments designed for minimally invasive cardiac surgery are used including needle drivers, scissors and knot pushers and an Endo Close trocar site closure device (Medtronic Minimally Invasive Therapies, Minneapolis, Minnesota, USA). We have performed aortic occlusion with either the Chitwood clamp (used in this presentation) or an endoaortic balloon as the safety of both methods has been demonstrated (14). Percutaneous Bio-Medicus arterial and venous cannulae (Medtronic, Minneapolis, Minnesota, USA) are utilized for extracorporeal circulation. Intraoperative TEE is employed both before and after completion of the reconstruction to assess position and motion of the reconstructed septum and presence of residual septal defects. A 30° high definition videoscope may be used to improve visualization.
Procedure
Arterial and central venous access is obtained and general anesthesia is induced. A TEE probe is inserted and the patient is placed supine with a sheet roll beneath the right hemithorax providing a 20–30° elevation (Figure 1).

The right arm is tucked in a slightly posterior sling and the patient is prepared and draped from chin to knees.
Access to the jugular and femoral vessels must be available within the prepared surgical field for subsequent percutaneous bicaval venous cannulation by the surgeon. Ultrasound-guided micropuncture access to the right internal jugular and common femoral veins is performed as well as open surgical access of the right common femoral artery. Alternatively, we have had good success with totally percutaneous cannulation utilizing the preclose technique in which 2–3 Perclose ProGlide devices (Abbott Vascular, Redwood City, California, USA) are deployed prior to cannulation as previously described (16). A right anterior minithoracotomy is performed via a 4–6 cm incision in the inframammary crease. An Alexis atraumatic soft tissue retractor (Applied Medical, Rancho Santa Margarita, California, USA) is used for exposure, avoiding the use of a rigid metallic retractor. A small bore flexible insufflation tubing for CO2 is placed through the wound outside the soft tissue retractor. Single lung ventilation is instituted at this time (Figure 2).

A pericardiotomy is created lateral to the right atrium. A curvilinear incision is created to facilitate subsequent harvest of a pericardial patch should the need arise. A figure-of-8 suture (2-0 silk) is placed in the central tendon of the right hemidiaphragm and exteriorized through the right anterior costophrenic sulcus through a small stab wound in the right upper quadrant of the abdomen using an Endo Close suture passer. This suture will later be retracted to provide exposure of the inferior vena cava (Figure 3).

A heparin bolus (300 units/kg) is then administered to achieve an activated clotting time of at least 450 s and the venous and arterial cannulas are inserted (21 French percutaneous cannula in the right internal jugular vein, a 29 French percutaneous cannula into the retrohepatic cava from the groin and a 21 French cannula directly into the femoral artery through a 5-0 polypropylene purse string suture). A long 7 Fr cardioplegia cannula (Sorin Group, Milan, Italy) is placed through a pledgeted 4-0 polypropylene purse string suture on the ascending aorta. Once an adequate activated clotting time has been confirmed, cardiopulmonary bypass is initiated with kinetically assisted drainage and the temperature is allowed to drift. After the right atrium collapses, the surgeon verifies that the venous cannulae are positioned peripheral to the respective atriocaval junctions. A large right angled clamp is then carefully passed around the cavae to encircle them with umbilical tape placed through red rubber snares (Figures 4,5).


The Chitwood aortic clamp is then inserted through a separate stab incision in the right axilla. The aorta is then clamped and 1 liter of cold blood cardioplegia (4:1) is administered antegrade into the aortic root. The cavae are then snared to occlusion (Figure 6).

A right atriotomy is performed and the edges are retracted with 2-0 silk stay sutures to further expose the ASA which is then resected (Figures 7,8).


If primary closure of the defect is not feasible, a pericardial patch is harvested and cut to the proper size and shape. The handling characteristics of the patch are improved if it is pretreated with 2% glutaraldehyde fixative. The patch is then sewn to the edges of the intact atrial septum with the smooth surface oriented towards the left atrium (Figure 9).

Prior to final closure of the suture line, the left atrium is meticulously deaired by repeatedly inflating the left lung and instilling saline into the left atrium. After completion of patch closure, the left atrium is transseptally aspirated to evacuate any retained air. Once the deairing maneuvers and repair are satisfactory, the aortic cross clamp is released and the aortic root vent is placed on suction. The atriotomy is closed in two layers of 3-0 polypropylene and the caval snares are released. Cardiopulmonary bypass is then weaned and TEE is performed to assess integrity of the repair (Figure 10).

The cannulae are then removed, placing a pledgeted 3-0 polypropylene horizontal mattress suture on a medium half-circle needle about each of the venous cannula tracts as they are removed. The arterial cannula site is clamped and directly repaired with interrupted 5-0 polypropylene sutures. Protamine sulfate is then administered. If there is any concern for pericardial herniation through the pericardial defect, it is partially closed with a polytetrafluorethylene patch. Liposomal bupivacaine suspension (Pacira Pharmaceuticals, Parsippany, New Jersey, USA) is injected for intercostal nerve block and local tissue infiltration around all incisions and chest drains. Two right pleural drains are placed and the wounds are then closed.
Role of team members
The operative team consists of the primary and assistant surgeons, anesthesiologist, echocardiographer, surgical assistant, surgical technician and circulating nurse.
Results
Post-operative management
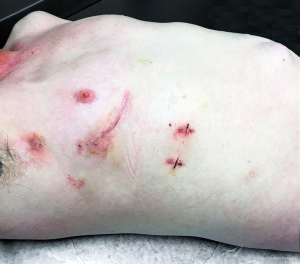
After minimally invasive cardiac surgery, patients are admitted to the cardiovascular intensive care unit overnight and are typically transferred to the stepdown unit the next morning. As in this case, discharge home on as early as the second postoperative day is often possible. Oral anticoagulation or dual antiplatelet therapy may be started as soon as oral intake is possible, provided that removal of the chest drains is anticipated in the first 24 hours and hemostasis is satisfactory, and is maintained for three months postoperatively (Figure 11).
Tips, tricks and pitfalls
To prevent major vascular injury during insertion of cannulae, a stiff wire such as a Lunderquist (Cook Medical, Bloomington, Indiana, USA) or Amplatz (Boston Scientific, Natick, Massachusetts, USA) is used under fluoroscopic and/or echocardiographic guidance. Use of rigid retractors on the minithoracotomy is associated with intercostal nerve compression and increased postoperative pain; therefore, atraumatic soft tissue retraction is used and provides adequate exposure. Circumferential caval control can be most safely and expeditiously accomplished after initiation of cardiopulmonary bypass when the right atrium is decompressed and visualization is much improved. When using the Chitwood clamp, great care is taken in visualizing the insertion and application to avoid injury to the pulmonary artery, an event that is likely to require conversion to sternotomy for repair. When selecting patch material, the use of autologous pericardium is preferred for its low thrombogenicity and infection resistance. Postoperative patch-related embolic events are further prevented with 3 months of anticoagulation or dual antiplatelet therapy as an alternative. Care is taken during patch harvesting to identify the location of the phrenic nerve. To prevent residual bulging or excessive motion of the reconstructed interatrial septum, the patch is intentionally downsized relative to the defect by approximately 25%. Air emboli are prevented by a variety of techniques. First, the operative field is flooded with carbon dioxide. Although it may be technically possible to avoid the use of cardioplegia, it is administered to provide a motionless field that prevents air trapping within the left atrium. Furthermore, aggressive suction within the left atrium is avoided to maintain the blood-air interface as high as feasibly possible. Immediately prior to closure of the suture line, the left lung is inflated several times and saline is used as needed to flush all air from the left atrium. After patch closure is complete, a 21 gauge needle is used for transseptal aspiration of the left atrium (avoiding the patch) to evacuate any residual air. The triangle of Koch is identified if the defect extends towards the atrioventricular groove to prevent atrioventricular node injury. If harvest of the pericardial patch results in a large defect, a patch closure is performed to prevent cardiac herniation.
Conclusions
ASAs can be safely repaired using minimally invasive techniques. It avoids a median sternotomy and is associated with shorter hospital length of stay and overall duration of recovery. A technically satisfactory repair can be performed consistently and safely. Peripheral cannulation for cardiopulmonary bypass is essential to maximize exposure through a limited incision. Most patients with an ASA will be candidates for minimally invasive repair as there are few contraindications.
Acknowledgements
Merry Crow and Chris Hibbler for assistance with surgical instrumentation; Kay Golden for surgical assistance, Jayson Powell for perfusion support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethical committee. Written informed consent was obtained from the patient for publication. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Olivares-Reyes A, Chan S, Lazar EJ, et al. Atrial septal aneurysm: a new classification in two hundred five adults. J Am Soc Echocardiogr 1997;10:644-56. [PubMed]
- Ghosh S, Ghosh AK, Ghosh SK. Patent foramen ovale and atrial septal aneurysm in cryptogenic stroke. Postgrad Med J 2007;83:173-7. [PubMed]
- Mügge A, Daniel WG, Angermann C, et al. Atrial septal aneurysm in adult patients. A multicenter study using transthoracic and transesophageal echocardiography. Circulation 1995;91:2785-92. [PubMed]
- Mattioli AV, Aquilina M, Oldani A, et al. Atrial septal aneurysm as a cardioembolic source in adult patients with stroke and normal carotid arteries. A multicentre study. Eur Heart J 2001;22:261-8. [PubMed]
- Hanley PC, Tajik AJ, Hynes JK, et al. Diagnosis and classification of atrial septal aneurysm by two-dimensional echocardiography: report of 80 consecutive cases. J Am Coll Cardiol 1985;6:1370-82. [PubMed]
- Belkin RN, Hurwitz BJ, Kisslo J. Atrial septal aneurysm: association with cerebrovascular and peripheral embolic events. Stroke 1987;18:856-62. [PubMed]
- Schneider B, Hanrath P, Vogel P, et al. Improved morphologic characterization of atrial septal aneurysm by transesophageal echocardiography: relation to cerebrovascular events. J Am Coll Cardiol 1990;16:1000-9. [PubMed]
- Mas JL, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001;345:1740-6. [PubMed]
- Wu LA, Malouf JF, Dearani JA, et al. Patent foramen ovale in cryptogenic stroke: current understanding and management options. Arch Intern Med 2004;164:950-6. [PubMed]
- Windecker S, Wahl A, Nedeltchev K, et al. Comparison of medical treatment with percutaneous closure of patent foramen ovale in patients with cryptogenic stroke. J Am Coll Cardiol 2004;44:750-8. [PubMed]
- Hung J, Landzberg MJ, Jenkins KJ, et al. Closure of patent foramen ovale for paradoxical emboli: intermediate-term risk of recurrent neurological events following transcatheter device placement. J Am Coll Cardiol 2000;35:1311-6. [PubMed]
- Homma S, Sacco RL, Di Tullio MR, et al. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002;105:2625-31. [PubMed]
- Orgera MA, O'Malley PG, Taylor AJ. Secondary prevention of cerebral ischemia in patent foramen ovale: systematic review and meta-analysis. South Med J 2001;94:699-703. [PubMed]
- Atluri P, Goldstone AB, Fox J, et al. Port access cardiac operations can be safely performed with either endoaortic balloon or Chitwood clamp. Ann Thorac Surg 2014;98:1579-83; discussion 1583-4. [PubMed]
- Naficy S, Klemis JE, Gubin SS, et al. Pre-repair TEE. Asvide 2016;3:030. Available online: http://www.asvide.com/articles/781
- Ramponi F, Yan TD, Vallely MP, et al. Total percutaneous cardiopulmonary bypass with Perclose ProGlide. Interact Cardiovasc Thorac Surg 2011;13:86-8. [PubMed]
- Naficy S, Klemis JE, Gubin SS, et al. Positioning, instrumentation and cannulation. Asvide 2016;3:031. Available online: http://www.asvide.com/articles/782
- Naficy S, Klemis JE, Gubin SS, et al. Pericardiotomy. Asvide 2016;3:032. Available online: http://www.asvide.com/articles/783
- Naficy S, Klemis JE, Gubin SS, et al. Caval dissection and control. Asvide 2016;3:033. Available online: http://www.asvide.com/articles/784
- Naficy S, Klemis JE, Gubin SS, et al. Placement of cardioplegia catheter. Asvide 2016;3:034. Available online: http://www.asvide.com/articles/785
- Naficy S, Klemis JE, Gubin SS, et al. Clamp placement and cardioplegic arrest. Asvide 2016;3:035. Available online: http://www.asvide.com/articles/786
- Naficy S, Klemis JE, Gubin SS, et al. Atriotomy and septal inspection. Asvide 2016;3:036. Available online: http://www.asvide.com/articles/787
- Naficy S, Klemis JE, Gubin SS, et al. Resection of atrial septal aneurysm. Asvide 2016;3:037. Available online: http://www.asvide.com/articles/788
- Naficy S, Klemis JE, Gubin SS, et al. Patch preparation and septal reconstruction. Asvide 2016;3:038. Available online: http://www.asvide.com/articles/789
- Naficy S, Klemis JE, Gubin SS, et al. Post-repair TEE. Asvide 2016;3:039. Available online: http://www.asvide.com/articles/790
Cite this article as: Naficy S, Klemis JE, Gubin SS, Funderburg WR, Craig JM. Minimally invasive repair of fenestrated atrial septal aneurysm. J Vis Surg 2016;2:21.