Thoracoabdominal aortic aneurysm (extent II) repair in a patient with systemic vasculitis
Introduction
Open repair of thoracoabdominal aortic aneurysm (TAAA) is a challenging procedure due to its surgical invasiveness and remarkable morbidity and mortality.
Recent advances in endovascular treatment of aortic aneurysm provide ways to overcome the anatomic limitation of repair of TAAA. The short-term and mid-term results are favorable and promising (1-3). However, endovascular treatments for TAAA are technically demanding, and currently, they are being performed only at limited centers throughout the world. A time span of several days for the production of a customized stent device for the procedure is another obstacle that prevents the universal use of endovascular treatment in emergent or urgent settings. Moreover, significant rates of endoleaks and re-intervention, high late mortality (aorta-related or non-aorta-related), and lack of long-term data are the problems with endovascular treatment of TAAA, which need to be solved in the near future (1-3).
During recent decades, the results of TAAA open repair have improved significantly due to the refinement of surgical strategy and the improvement in intra- and post-operative management. Experienced surgeons and centers report operative mortality rates of 5–8% and paraplegia rates of 3–8% (4-7). Furthermore, open surgical repair of TAAA is the best option for patients with connective tissue disorders or systemic vasculitis, who are not good candidates for endovascular treatment (8). Hence, young vascular surgeons need to learn the technique of open surgical repair of TAAA in addition to endovascular skills.
Patient selection and work-up
The patient in the video clip (Figure 1) is a 42-year-old male with known hypertension. He had suffered DeBakey type III acute aortic dissection 9 months ago and was receiving follow-up after medical treatment. Increasing intensity of abdominal pain (left umbilical area, dull nature) led to the chest and abdominal computed tomography (CT) examination. A rapidly growing TAAA (Figure 2) was detected.

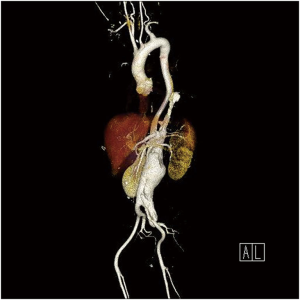
On preoperative work-ups, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels were increased. The patient and his brother had vitiligo. Brain magnetic resonance angiography (MRA), which is not a routine evaluation tool in young patients of our hospital, revealed two large aneurysms (12 and 10 mm in diameter, respectively) of the right vertebral artery.
After rheumatology consultation and the related work-ups, oral steroid treatment was started under the diagnosis of unspecified systemic vasculitis. After a 1-month treatment with oral steroid (Sorondo 10 mg bid), CRP and ESR levels were almost within the normal range.
After weighing the relative risk of the TAAA repair-first strategy as compared to the brain aneurysm-first treatment, we decided to proceed with the TAAA open repair first under the consent and complete understanding of the patient and his family.
Pre-operative preparation
A double-lumen endotracheal tube was inserted. Arterial pressure monitoring cannulas were inserted in the right radial artery and the right dorsalis pedis artery. A cerebrospinal fluid (CSF) drain was inserted to measure the CSF pressure and to remove the fluid. The CSF pressure was maintained within the 10–12 mmHg range. A cell-saving device was prepared. Somatosensory- or motor-evoked potential monitoring was not used.
The patient was placed in a modified right lateral decubitus position (shoulders at 90 degrees from the horizontal plane and hips at 45 degrees) using a bean-bag device and position tools.
Equipment preference card
Mesenteric artery perfusion catheters: 15 Fr. DLP retrograde cannula (Medtronic, Dublin, Ireland) or 10 Fr. Gundry retrograde cannula (Medtronic Inc., Minneapolis, MN, USA). Segmental artery blockers: 3 or 4 Fr. Occlusion catheter (LeMaitre Vascular, MA, USA). Main aortic graft: Gelweave Coselli thoracoabdominal graft (Vascutek Ltd, Inchinnan, Scotland). Right iliac artery graft: Hemashield 14 mm graft (Maquet, Rastatt, Germany).
Procedure
The left thoracoabdominal incision was made, and the pleural cavity was entered through the 6th intercostal space. Lung adhesion to the proximal descending thoracic aorta (DTA) was encountered. Once the space required for performing a longitudinal aortotomy was obtained, we avoided performing additional adhesiolysis to prevent lung injury.
Aortic clamp sites on the proximal descending aorta, mid-descending aorta, distal descending aorta, and both iliac arteries were prepared using umbilical tapes.
After full heparinization, partial cardio-pulmonary bypass (CPB) was instituted using the left femoral artery and femoral vein cannula. Distal perfusion and upper body hypertension during aortic cross-clamping were controlled by reduction of the preload and increase of lower body perfusion. The advantage of partial CPB is safe control of volume loss during massive bleeding by using the pump suckers and reservoir. Permissive mild hypothermia (32–34 °C, nasopharyngeal) was maintained.
For this patient with systemic vasculitis, Gelweave Coselli 22 mm thoracoabdominal graft (Vascutek Ltd, Inchinnan, Scotland) was chosen. In patients with connective tissue disorder or systemic vasculitis, separate anastomoses of mesenteric arteries are preferred over island patch anastomosis to prevent the development of late pseudoaneurysm at the suture sites (10).
To minimize the ischemic time of organs, sequential aortic clamping and aortic opening were performed. Throughout the whole procedure, the expected hypotension and acidosis after removal of the aortic cross-clamp was managed with aggressive fluid resuscitation and sodium bicarbonate infusion (11).
With respect to the segmental arteries, T9 and T10 segmental arteries were reattached to the main graft and the other segmental arteries were oversewed. Before reattachment of segmental arteries, 3-Fr. and 4-Fr. occlusion catheters (LeMaitre Vascular, MA, USA) were inserted into the targeted T9 and T10 segmental arteries to control the back-bleeding, and thus improve spinal cord perfusion.
With respect to the mesenteric arteries, 15-Fr. balloon perfusion catheters (DLP retrograde cannula, Medtronic Inc., Minneapolis, MN, USA) were inserted and directed into the ostia of the celiac artery and superior mesenteric artery for continuous perfusion with oxygenated blood. For renal protection, cold (4 °C) crystalloid solution was infused intermittently (300 cc per kidney, every 10 min) through the 10-Fr. balloon perfusion catheters (Gundry retrograde cannula, Medtronic Inc., Minneapolis, MN, USA). The crystalloid solution was made of lactated Ringer’s solution 1,000 cc, solumedrol 125 mg/L and mannitol 25 mg/L (12).
In this patient, the dissection was extended to the left common iliac artery and size discrepancy between the aortic bifurcation and the 22 mm main graft caused problems in determining the location of distal anastomosis. Instead of using another bifurcated abdominal graft for aorto-iliac anastomosis and a separate graft for the left internal iliac artery, the distal anastomosis was made on the left common iliac artery, which was dilated.
Following the distal anastomosis, separate anastomoses of mesenteric arteries were performed in the following sequence: right renal artery, superior mesenteric artery, celiac artery, and left renal artery. Finally, separate anastomosis of the right common iliac artery was performed. Hemashield 14 mm graft (Maquet, Rastatt, Germany) was used for the anastomosis. To solve the problem of redundancy of the abdominal aortic graft, the redundant portion was resected and re-anastomosed.
CPB time for the procedure was 195 min.
Post-operative management
Mean arterial blood pressure was continuously monitored and maintained between 80 and 90 mmHg. CSF pressure was also continuously monitored and maintained within the 10–12 mmHg range. After confirming the patient’s mental recovery and leg movements without impairment, CSF pressure was allowed to equilibrate at the upper level of 15 mmHg without CSF drainage. The CSF drainage catheter was removed on postoperative day 2.
The patient was discharged from the hospital on postoperative day 15.
Tips, tricks, and pitfalls
In the absence of an urgent or emergent state, it is reasonable to delay the open repair in a patient with systemic vasculitis until the acute inflammatory state is treated or quiescent. Disease activity is evaluated by checking the ESR and CRP levels.
In patients with connective tissue disorder or systemic vasculitis, the aortic wall tissues are very fragile and prone to easy tearing. Especially in this patient, the aortic tissue around the T9 and T10 segmental arteries was very weak and fragile. Under these circumstances, the suture bites have to be deeper and wider instead of shallow and narrow bites.
For measuring the desired length of the aortic graft, extendable grafts, like Gelweave graft (Vascutek Ltd, Inchinnan, Scotland), have to be stretched out to cut a graft of the desired length.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Greenberg R, Eagleton M, Mastracci T. Branched endografts for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg 2010;140:S171-8. [PubMed]
- Verhoeven EL, Katsargyris A, Bekkema F, et al. Editor's Choice - Ten-year Experience with Endovascular Repair of Thoracoabdominal Aortic Aneurysms: Results from 166 Consecutive Patients. Eur J Vasc Endovasc Surg 2015;49:524-31. [PubMed]
- Chuter TA, Rapp JH, Hiramoto JS, et al. Endovascular treatment of thoracoabdominal aortic aneurysms. J Vasc Surg 2008;47:6-16. [PubMed]
- Safi HJ, Estrera AL, Miller CC, et al. Evolution of risk for neurologic deficit after descending and thoracoabdominal aortic repair. Ann Thorac Surg 2005;80:2173-9; discussion 2179. [PubMed]
- Kouchoukos NT, Kulik A, Castner CF. Outcomes after thoracoabdominal aortic aneurysm repair using hypothermic circulatory arrest. J Thorac Cardiovasc Surg 2013;145:S139-41. [PubMed]
- LeMaire SA, Price MD, Green SY, et al. Results of open thoracoabdominal aortic aneurysm repair. Ann Cardiothorac Surg 2012;1:286-92. [PubMed]
- Schepens MA, Heijmen RH, Ranschaert W, et al. Thoracoabdominal aortic aneurysm repair: results of conventional open surgery. Eur J Vasc Endovasc Surg 2009;37:640-5. [PubMed]
- Coady MA, Ikonomidis JS, Cheung AT, et al. Surgical management of descending thoracic aortic disease: open and endovascular approaches: a scientific statement from the American Heart Association. Circulation 2010;121:2780-804. [PubMed]
- Kim JH. Video clip presenting thoracoabdominal aortic aneurysm repair in a patient with systemic vasculitis. Asvide 2016;3:075. Available online: http://www.asvide.com/articles/828
- LeMaire SA, Carter SA, Volguina IV, et al. Spectrum of aortic operations in 300 patients with confirmed or suspected Marfan syndrome. Ann Thorac Surg 2006;81:2063-78; discussion 2078. [PubMed]
- Wong DR, Parenti JL, Green SY, et al. Open repair of thoracoabdominal aortic aneurysm in the modern surgical era: contemporary outcomes in 509 patients. J Am Coll Surg 2011;212:569-79; discussion 579-81. [PubMed]
- Lemaire SA, Jones MM, Conklin LD, et al. Randomized comparison of cold blood and cold crystalloid renal perfusion for renal protection during thoracoabdominal aortic aneurysm repair. J Vasc Surg 2009;49:11-9; discussion 19. [PubMed]
Cite this article as: Kim JH. Thoracoabdominal aortic aneurysm (extent II) repair in a patient with systemic vasculitis. J Vis Surg 2016;2:35.


