Management of malignant pleural effusions in patients with trapped lung with indwelling pleural catheter: how to do it
Introduction
All thoracic surgeons worldwide are members of multidisciplinary cancer teams, so regularly treat patients with malignant pleural effusion (MPE) in their daily clinical practice. MPE is a well-known sign of an end-stage cancer and affects the quality of life of these patients. The increased pleural fluid production is due to the increased vascular permeability, or to the reduced reabsorption by lymphatic vessels. The primary goal in the management of MPE should be a soothing treatment with the palliation of symptoms (1). Pleurodesis may be accomplished with chemical irritation of the pleura and represents the commonest treatment of MPE with palliative intent. Pleurodesis may be achieved through a chest drainage placement or a video-assisted thoracic surgery (VATS) procedure. The uniportal VATS talc poudrage is considered the gold standard of care for fit patients, while talc slurry (through the chest drainage) is reserved to those patients with important comorbidities not tolerating a surgical procedure (2). However, if the lung remains trapped after fluid evacuation or if the daily fluid output after chest tube insertion is major than 300 mL/day, the talc pleurodesis is likely to fail. Therefore, in those patients who are unfit for pleurodesis (low performance status or comorbidity), or with a recurrent MPE after chemical pleurodesis, or with trapped lung, the outpatient intermittent drainage through a subcutaneous tunnelled indwelling pleural catheter (IPC) effectively relieved dyspnoea without complications (3). We report our experience with the IPC placements in MPE patients unfit for other procedures.
Patient selection and workup
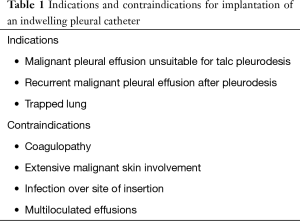
Before IPC insertion, a therapeutic thoracentesis should be performed with cytological examination of the effusion. In addition, thoracentesis allows to evaluate the response to drainage, confirming whether the lung is able to re-expand and how rapidly the MPE reaccumulates. If the lung is unable to re-expand sufficiently, few therapeutic options other than IPC exists. As expected, the IPC is unnecessary if the fluid does not reaccumulate following a large volume thoracentesis or if symptoms are not improved by the procedure. Finally, patients whose life expectancy is very short (days or few weeks) should better undergo other treatment options such as intermittent thoracentesis or placement of traditional (not-tunnelled) small-bore chest drainages. The indications for IPC implantation (Table 1) are a MPE considered unsuitable for talc pleurodesis, a recurrent MPE after pleurodesis, or a trapped lung (3). The contraindications to IPC placement (Table 1) include coagulopathy, extensive malignant skin involvement, infection over the site of insertion, and multiloculated or septated effusions that would not drain even with an IPC in place (4). A written informed consensus must be obtained from all patients before each surgical procedure.
Full table
Pre-operative preparation
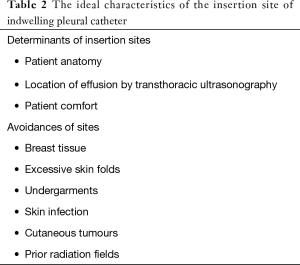
The IPC placement does not require inpatient admission and may be performed bedside anywhere there is patient monitoring and a sterile environment: the intensive care unit, the ambulatory procedure centre, or (obviously) the operating room. Although local anaesthesia is standard, the type and dosages of other medications (e.g., intravenous analgesics or sedatives) could differ. There is no a single ideal insertion site. The determinants of the insertion site of IPC (Table 2) include patient anatomy, location of the effusion by transthoracic ultrasonography, and, not least, patient comfort. The IPC insertion sites should avoid breast tissue, excessive skin folds, undergarments, skin infection, cutaneous tumours, and prior radiation fields. During placement, patients may be positioned semirecumbent with their ipsilateral arm raised above their head, or, alternatively, in the lateral decubitus position with the affected side up. In addition to the chest physical examination, radiographic imaging may identify the most appropriate entry site. Transthoracic ultrasonography examination can be particularly helpful in more complex fluid collections, in metastatic involvement of the pleura, or in the presence of adhesions (4).
Full table
Equipment preference card
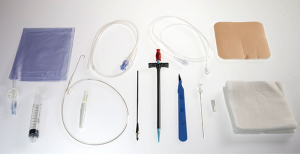
Two years ago we adopted the Relief® (Figure 1) system (Med-Italia Biomedica S.r.l., Genova IT). The IPC set contains all the gear needed to place the drainage (except for the local anaesthesia and the suture) and this is useful when the drainage needs to be placed in other units avoiding the risk of forgetting material. The set (Figure 2) contain a 65-cm 15.5 Fr fenestrated silicone catheter, a safety scalpel, a guide wire introducer with needle, a 10-mL luer lock male Syringe, a 60-cm J-tip guide wire, a valve 16-Fr peel-away introducer, a metal tool for tunnelization, a needle foam stop, and a catheter insertion stylet. The manoeuvre of IPC placement, performed under local anaesthesia, requires only the presence of a surgeon and a nurse. In addition to the supplied kit, the IPC insertion required supplies as antiseptic solution (e.g., Chlorhexidine, Povidone Iodine), personal protective equipment (e.g., hat, mask with face shields, sterile gown, and gloves), sterile drapes, and ultrasound.

Procedure
Ultrasonography has been used to allow the surgeon to locate the MPE and to confirm the site of insertion. The entry site and the counter incision were marked with an indelible marker (Figure 3). After preprocedural planning, the skin was cleansed with an antiseptic solution over an extended surface that allows a sterile margin of at least 10 cm around the IPC entry and exit sites, as well as along the entirety of the tunnel. The broad sterile drape was applied. Lidocaine 2% 20 mL was used to inoculate the skin and the soft tissues up to the level of the parietal pleura and along the length of the tunnel. On the track identified during the initial anaesthesia, a guide needle was then advanced while aspirating. Once pleural fluid was seen in the syringe, the guide wire was passed through the needle into the pleural space, and then the needle was removed. Two incisions were made at the entry site (i.e., over the pleural entry site) and exit sites (where the catheter will exit the skin). We suggest to limit the skin incision at the planned exit site (maximum 1 cm) in order to barely allow the catheter to exit the skin, minimizing the risk of dislocation of the catheter. The catheter then is passed from the skin exit site toward the pleural entry site. This manoeuvre is allowed by the tunnelling tool. The plastic dilator tool is passed along the guide wire in order to create the route for the introducer. Once entered the pleura, the dilator and the guide wire were removed and substituted with the peel-away introducer. The IPC was rapidly advanced through the sheath to minimize the flow of air into the pleural space. Once the entire catheter was advanced through the sheath, it was peeled apart and removed. The incisions were closed and the catheter was secured to the skin with a suture material. Once the IPC was secured, the pleural space was drained. The drainage line was connected to the one-way valve at the end of the IPC. Pleural fluid was removed until the patient developed symptoms such as cough, pain, or dyspnea. The drainage system was then disconnected and the plastic valve cap provided was secured onto the valve. A foamy drain sponge was placed over the catheter, in order to prevent decubitus. A chest radiograph was performed following catheter insertion to ensure the adequate placement and the absence of complications. Once the roentgenogram was reviewed by the surgeon, the patient could be discharged home (3-5).

Role of team members
Patients with IPC benefit from the continuity of care and the accessibility to a team experienced in the long-term management, besides drainage insertion. This goal often was achieved in the context of multidisciplinary cancer teams. Ideally, patients and their families received training on the method of catheter drainage, application of dressings, sterile technique, and indications for reaching health care providers if problems arise (4). The family members or friends and others personnel performed the chronic management of the IPC. Community nurses involved in each patient’s care underwent a vigorous training programme with thoracic nurse (6). When the patient was not in a facility or the home nursing not arranged, initial education included videos, reading materials, and observation of drainage by members of the health care team was performed (7).
Post-operative management
A routine follow-up visit was generally scheduled for all patients two weeks after insertion. At this time, a chest roentgenogram was performed and symptoms, concerns, and complications were addressed. Dyspnoea control was described with a three-point scale. The incisions are inspected and sutures are removed. After this visit, patients are seen on an as-needed basis, if problems or new symptoms arise, or if IPC removal is indicated. Follow-up and support by well-trained district nurses was scheduled on an outpatient basis and at least a weekly visit was planned. At least weekly fluid evacuation was recommended, until less than 500 mL was obtained at a time, in this case more time should be elapsed between evacuations. The success or failure of pleurodesis was assessed using the American Thoracic Society guidelines (8). Mainly, a complete successful pleurodesis was defined as a long-term symptoms relief, with the absence of fluid reaccumulation at the chest X-ray whereas a partial successful pleurodesis indicates the diminution of dyspnea related to effusion, with only partial reaccumulation of fluids, without the need for further thoracentesis. When no drainage took place for one month and a chest roentgenogram showed no significant MPE, the possible removal under local anaesthesia was offered to the patient.
Tips, tricks and pitfalls
The IPC is an effective method for controlling those patients who display a recurrent symptomatic MPE. Nevertheless, talc slurry or thoracoscopic poudrage are the method of choice to achieve pleurodesis, with over 90% success rates, if the lung can expand. On the other hand, uniportal VATS pleurodesis is the gold standard for effusions with unclear aetiology and non-diagnostic pleural fluid study. The presence of foreign material within the pleural space stimulates an inflammatory reaction, and the vacuum drainage bottles connected to the catheter encourage lung re-expansion with the obliteration of the pleural space. The IPC is an excellent choice when the patients have trapped lungs. Potential complications of pleural catheters (infections and dislocations) are uncommon. Our experience (more than 15 years in the use of IPC) suggests in rapidly recurring MPE that the early implantation is preferred to the repeated needle thoracentesis (3,9-11). We observed that patients may benefit from intermittent fluid evacuation, ambulation, and self-care and the risk of needle thoracentesis-associated pneumothorax is avoided. In a previous paper, we also observed a pleurodesis rate in line with data reported in literature (3). The variable success rate of pleurodesis relates to different types of malignancy, which have different pathogenesis leading to the effusion, such as a pleural spreading for mesothelioma or mediastinal node metastases and occlusion of the lymphatic for breast cancer, or other factors such as the degree of lung entrapment by tumour growth (7,12-16). The cost of an IPC may appear as an issue, mainly due to the cost of the device and the single-use vacuum bottles. However, in spite of these factors, IPC has been found to be the most cost-effective method since the duration of hospital stay is shorter or avoided (17-22).
In conclusion, the treatment of recurrent MPE with an IPC reduces symptoms and improves quality of life in patients with end-stage cancers. The complication rate is low; therefore, the IPC can be easily managed at home. The IPC is safe, easy to place and effective for the palliation of MPE. It could help the clinical need of the thoracic surgeons and the other members of a multidisciplinary cancer team.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zarogoulidis K, Zarogoulidis P, Darwiche K, et al. Malignant pleural effusion and algorithm management. J Thorac Dis 2013;5 Suppl 4:S413-9. [PubMed]
- Filosso PL, Sandri A, Felletti G, et al. Preliminary results of a new small-bore percutaneous pleural catheter used for treatment of malignant pleural effusions in ECOG PS 3-4 patients. Eur J Surg Oncol 2011;37:1093-8. [Crossref] [PubMed]
- Bertolaccini L, Viti A, Gorla A, et al. Home-management of malignant pleural effusion with an indwelling pleural catheter: ten years experience. Eur J Surg Oncol 2012;38:1161-4. [Crossref] [PubMed]
- Myers R, Michaud G. Tunneled pleural catheters: an update for 2013. Clin Chest Med 2013;34:73-80. [Crossref] [PubMed]
- Bertolaccini L, Viti A, Terzi A. Placement of an indwelling pleural catheter in a patient with a right malignant pleural effusion. Asvide 2016;3:088. Available online: http://www.asvide.com/articles/836
- Efthymiou CA, Masudi T, Thorpe JA, et al. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009;9:961-4. [Crossref] [PubMed]
- Putnam JB Jr, Walsh GL, Swisher SG, et al. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg 2000;69:369-75. [Crossref] [PubMed]
- Schrader JM, Ferson PF. Managing recurrent pleural effusions with an indwelling pleural catheter. JAAPA 2009;22:27-8, 33-4. [Crossref] [PubMed]
- Bertolaccini L, Zamprogna C, D'Urso A, et al. The treatment of malignant pleural effusions: the experience of a multidisciplinary thoracic endoscopy group. Tumori 2003;89:233-6. [PubMed]
- Bertolaccini L, Zamprogna C, D'Urso A, et al. P-807 Outpatient management of malignant pleural effusions. Lung Cancer 2005;49:S331. [Crossref]
- Bertolaccini L, Zamprogna C, Barberis L, et al. Malignant pleural effusions: review of treatment and our experience. Rev Recent Clin Trials 2007;2:21-5. [Crossref] [PubMed]
- Putnam JB Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999;86:1992-9. [Crossref] [PubMed]
- Ahmed L, Ip H, Rao D, et al. Talc pleurodesis through indwelling pleural catheters for malignant pleural effusions: retrospective case series of a novel clinical pathway. Chest 2014;146:e190-4. [Crossref] [PubMed]
- Azzopardi M, Porcel JM, Koegelenberg CF, et al. Current controversies in the management of malignant pleural effusions. Semin Respir Crit Care Med 2014;35:723-31. [Crossref] [PubMed]
- Boshuizen RC, Burgers JA, van den Heuvel MM. Comments on predictors of clinical use of pleurodesis and/or indwelling pleural catheter therapy for malignant pleural effusion. Chest 2015;147:e232. [Crossref] [PubMed]
- Suzuki K, Servais EL, Rizk NP, et al. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol 2011;6:762-7. [Crossref] [PubMed]
- Olden AM, Holloway R. Treatment of malignant pleural effusion: PleuRx catheter or talc pleurodesis? A cost-effectiveness analysis. J Palliat Med 2010;13:59-65. [Crossref] [PubMed]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012;307:2383-9. [Crossref] [PubMed]
- Davies HE, Lee YC. Management of malignant pleural effusions: questions that need answers. Curr Opin Pulm Med 2013;19:374-9. [Crossref] [PubMed]
- Boshuizen RC, Onderwater S, Burgers SJ, et al. The use of indwelling pleural catheters for the management of malignant pleural effusion--direct costs in a Dutch hospital. Respiration 2013;86:224-8. [Crossref] [PubMed]
- Rial MB, Lamela IP, Fernández VL, et al. Management of malignant pleural effusion by an indwelling pleural catheter: A cost-efficiency analysis. Ann Thorac Med 2015;10:181-4. [Crossref] [PubMed]
- Penz ED, Mishra EK, Davies HE, et al. Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest 2014;146:991-1000. [Crossref] [PubMed]
Cite this article as: Bertolaccini L, Viti A, Terzi A. Management of malignant pleural effusions in patients with trapped lung with indwelling pleural catheter: how to do it. J Vis Surg 2016;2:44.


