Intrauterine diagnosed sternal cleft patient and her management
Introduction
Sternal cleft is a rare congenital anomaly which results from failure of fusion of the mesodermal sternal bars by 8 weeks of gestation (1-3). The rest of the sternum and its association with the ribs laterally usually develops normal. Complete and incomplete forms exist depending on the degree of separation. Upper or lower parts of the sternum may be involved, also unusually there may be a complete sternal non-union. Superior clefts are more common than inferior ones, but isolated central clefts are unlikely. That can be associated with many malformations (anterior cervical web, hemangiomatosis, central nervous system malformations, coloboma, and pectus excavatum) (4). It has been reported in mainly females (8:1) (3). An excavated midline thoracic defect and paradoxical chest wall movements with respiration is one of the pathognomonic symptom (4).
Clinically, three groups of sternal cleft can be described: first one is sternal cleft without associated anomalies. During embryonal development fusion starts from above. The defects either involve entire sternum or only the lower part. It can be V-shaped when it reaches the xiphoid process, or broad and U-shaped with a bony bridge joining the two edges, ending at the 3rd or 4th costal cartilages (5,6). This is reported in literature only about 100 patients (6). Sternal cleft can be diagnosed prenatally by ultrasonography, however, isolated sternal clefts can be difficult to diagnose (7). Normal skin and soft tissues almost always cover the bony defect. The heart pulsations and respiratory movements of the lung are obvious. These cases are usually asymptomatic, but dyspnea and recurrent pulmonary infections can present. Cardiac defects are rare in isolated sternal clefts and vascular malformations (e.g., craniofacial hemangiomas) can sometimes accompany. True ectopia cordis in which elevated heart anterior to the chest wall accompany to sternal cleft and Cantrell’s pentalogy in which the anomalous association of defects of the midline, at the supraumbilical abdominal wall, the lower sternum, the anterior diaphragm, the pericardium, with congenital intracardiac defects present are other two forms of the clinical presentation of sternal cleft (8).
Surgical correction is indicated after birth to protect vital intrathoracic organs, and potential injury to the intrathoracic structures could be the indication for surgery. Surgery is cosmetic in the absence of vital indications. Surgery is preferred in the newborn because the sternum has maximal flexibility and compression of underlying structures is minimal (4,9). Primary closure is the gold standard in the newborn period and early operation is necessary for a better surgical and cosmetic outcome. Isolated sternal cleft has a good postoperative prognosis (3). In older patients the decrease in chest volume with surgery may cause restriction (4).
Primary approximation, sliding or rotating chondrotomies, and reconstruction of the defects with the use of prosthetic grafts or flaps of bone, cartilage, autogenous tissue, or pectoralis major muscle are the main techniques used to correct sternal cleft (9). In adult patients with sternal cleft, chest wall rigidity and decreased space for intrathoracic organs must be considered (9). In older patients, autologous grafts are preferable due to less reaction and less infection risk (3).
Patient selection and workup
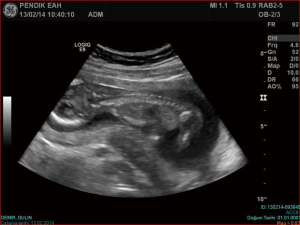
We hereby report our sternal cleft patient who is diagnosed intrauterinely (Figure 1) and followed up by our clinic. E.M.D was 5 days old, female patient when operated. She was transferred from newborn intensive care unit (ICU) of another hospital. Patient’s general condition was moderate. She had sternal cleft and multiple lesions on her mouth. She was extubated but hardly breathing.
Pre-operative preparation
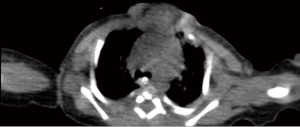
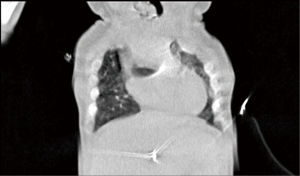
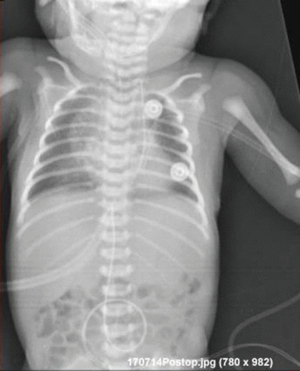
The patient was transferred from ICU of another hospital. She has been extubated. There were not any significant pathology rather than cleft and the mass. We evaluated the thorax CT (Figures 2,3) and transferred the patient to the operation room emergently because of respiratory distress (Figure 4).
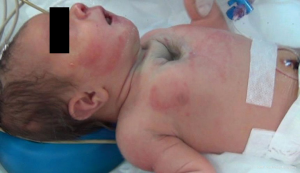
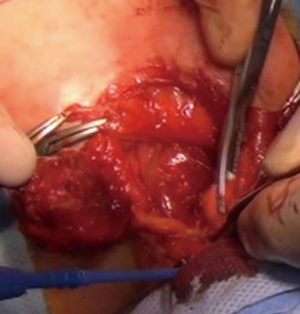
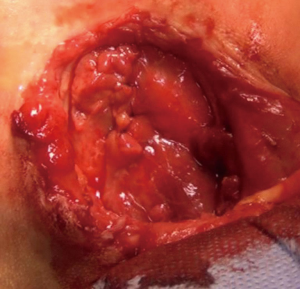
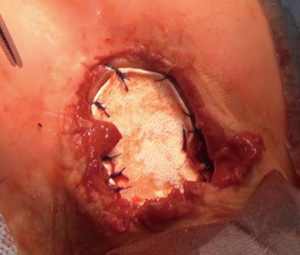
We operated the patient (Figure 5). There was a mass in the cleft and pericardial cyst beneath it (Figures 6,7). During the resection of the mass, we found out that there is a connection through the larynx. We resected the mass and pericardial cyst. Then, we sutured our gore-tex mesh between two sides of the sternum (Figure 8). We closed pericardium primarily by interrupted sutures. Operation time was 3 hours. Intravenous analgesia was performed during the operation and postoperatively. After our resection Ear Nose and Throat (ENT) doctors resected the laryngeal and oral part of the mass laryngoscopically. Plastic surgeons used pectoral muscles as flaps to cover up the space over our mesh. Informed consent was obtained from the family of the patient.

Post-operative management
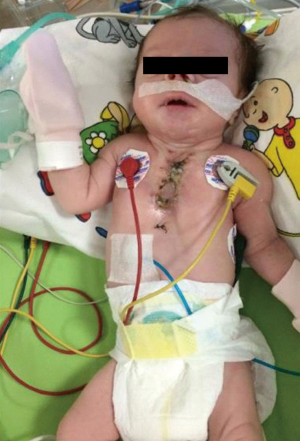
In postoperative day 15, patient was stable (Figures 9,10). All vital parameters and laboratory results were in the normal limits. She had only tachypnea and the reason was unknown. The mass pathology in the cleft was identified as congenital venous malformation and the mass in the larynx and the mouth was identified as rhabdomyoma. Patient stayed 3 months at the ICU due to recurrent pneumonia attacks. She kept entubated at that period due to respiratory distress. She was consulted ENT and they could not find any obstruction in any part of the larynx. Tracheostomy was performed for pulmonary toileting and avoiding from tracheal stenosis. She was discussed with pediatric pulmonologists for recurrent pneumonia attacks. They could not find any significant pathology. She was extubated at third month. She was transferred to the ward one month later and discharged from hospital with tracheostomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Schwabegger AH, editor. Congenital Thoracic Wall Deformities: Diagnosis, Therapy and Current Developments. Wien: Springer-Verlag, 2011.
- Kuru P, Ermerak NO, Bostanci K, et al. Reconstruction of sternal cleft with autologous cartilage graft in an adult. Asian Cardiovasc Thorac Ann 2015;23:591-2. [Crossref] [PubMed]
- Luthra S, Dhaliwal RS, Singh H. Sternal cleft--a natural absurdity or a surgical opportunity. J Pediatr Surg 2007;42:582-4. [Crossref] [PubMed]
- Biswas G, Khandelwal NK, Venkatramu NK, et al. Congenital sternal cleft. Br J Plast Surg 2001;54:259-61. [Crossref] [PubMed]
- Yavuzer S, Kara M. Primary repair of a sternal cleft in an infant with autogenous tissues. Interact Cardiovasc Thorac Surg 2003;2:541-3. [Crossref] [PubMed]
- Dòmini M, Cupaioli M, Rossi F, et al. Bifid sternum: neonatal surgical treatment. Ann Thorac Surg 2000;69:267-9. [Crossref] [PubMed]
- Rose NC, Coleman BG, Wallace D, et al. Prenatal diagnosis of a chest wall hamartoma and sternal cleft. Ultrasound Obstet Gynecol 1996;7:453-5. [Crossref] [PubMed]
- Cantrell JR, Haller JA, Ravıtch MM. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium, and heart. Surg Gynecol Obstet 1958;107:602-14. [PubMed]
- de Campos JR, Das-Neves-Pereira JC, Velhote MC, et al. Twenty seven-year experience with sternal cleft repair. Eur J Cardiothorac Surg 2009;35:539-41. [Crossref] [PubMed]
- Yuksel M, Kuru P, Ermerak NO, et al. The step-by-step procedure of the management of the patient with sternal cleft. Asvide 2016;3:100. Available online: http://www.asvide.com/articles/854
Cite this article as: Yuksel M, Kuru P, Ermerak NO, Kiyan G. Intrauterine diagnosed sternal cleft patient and her management. J Vis Surg 2016;2:48.