Video-assisted thoracoscopic surgery: pneumonectomy for synchronous primary lung malignancies
Introduction
Surgical treatment of non-small cell lung cancer (NSCLC) is optimal for early stage disease and is associated with reduced risk of local recurrence and improved long-term survival (1-5). Open surgical resection for NSCLC, involving thoracotomy and spreading of the ribs, is associated with increased postoperative pain and pulmonary complications when compared to minimally invasive surgical techniques, including video-assisted thoracoscopic surgery (VATS) (4,6-8). In addition to having similar lung and mediastinal dissection quality to thoracotomy procedures, benefits of VATS include: (I) reduced surgical trauma; (II) earlier return to normal daily activities; (III) better preservation of pulmonary function; and (IV) improved postoperative recovery leading to increased likelihood of completing an adjuvant chemotherapy regimen (4,6,8-15). Not unlike VATS lobectomy, researchers have reported improved long term survival for VATS pneumonectomy compared to open thoracotomy, despite the VATS group having more preoperative complications and a greater age (10,14,16,17). Though VATS procedures are more technically demanding, after advanced training using this approach and an initial learning curve, VATS may allow for more accurate dissection via enhanced illumination and magnification (4,6,11,18,19). Although rare, timely conversion to thoracotomy during VATS procedures may be necessary to avoid irreversible consequences. Reasons for conversion include uncontrolled bleeding, pleural adhesions, bronchial injury, and contralateral pneumothorax (5,8,11). As the proportion of converted cases decrease with increased surgical experience in VATS procedures, a simultaneous increase in mortality benefit is expected (20).
Multiple primary malignancies are a term used to define the presence of two or more separate primary malignancies in an individual patient (21). The development of multiple primary lung cancer (MPLC) is suggestive of continual exposure to risk factors for lung cancer. Patients have a 1–15% risk of developing MPLC per year (21,22). Previous studies have suggested that synchronous, central, ipsilateral upper and lower lobe malignancies are an indication for VATS pneumonectomy (8,20). A multidisciplinary team management approach of MPLC is optimal and primary surgical treatment or combined modality treatment strategies should be considered on a case by case basis (3).
We present a patient who underwent left VATS pneumonectomy for synchronous primary lung cancer and detail the technical aspects of the procedure (Figure 1).

Patient selection and workup
VATS is a surgical technique that has allowed for older patients, with more comorbidity, to be considered for surgical treatment when they otherwise may have been excluded (8,11,20). VATS pneumonectomy is a novel surgical treatment option and shares the same indications for pneumonectomy as traditional thoracotomy procedures. Contraindications for VATS pneumonectomy include: (I) extensive cancer spread to the chest wall or mediastinum; (II) complicated vascular reconstruction; and (III) lesions larger than 5 cm which may be difficult to extract thru a small incision without significant rib spreading (8). Because tumor compressibility varies, individual evaluation of patient candidacy for VATS pneumonectomy is required.
This study was exempt from review by the Institutional Review Board at the University of California, Davis. Informed consent was obtained from the patient. Our patient is a 71-year-old woman with a history of poorly controlled asthma, chronic pain, hypertension and diabetes who was incidentally found to have a left lower lobe nodule on workup for shoulder surgery. A separate mass was identified in the lingula. She reported mild dysphagia, wheezing and chronic cough productive of clear sputum but denied fevers, night sweats, and unintentional weight loss. Her performance status (Zubrod Score) was 1.
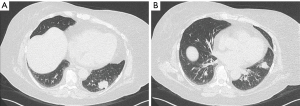
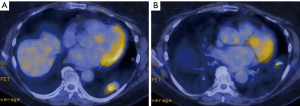
Preoperative computed tomography (CT) scan demonstrated a lower lobe mass with peribronchial soft tissue thickening and irregularly spiculated margins along the inferior lingular bronchus (Figure 2). Positron emission tomography (PET) scan showed a hypermetabolic left lower lobe mass with standardized uptake values (SUV) of 7.0, highly suspicious for primary lung cancer, and a second mass in the lingula with SUV of 2.9. There was no evidence of mediastinal or hilar hypermetabolic lymph nodes or signs of metastatic disease (Figure 3). CT-guided core needle biopsy of the lingular mass revealed adenocarcinoma positive for thyroid transcription factor 1 (TTF-1), which is a histological marker for lung development, and correlative for a primary lung tumor (24). Our patient’s myocardial perfusion scan was within normal limits and her pulmonary function tests (PFTs) revealed adequate function to tolerate a pneumonectomy with a forced expiratory volume in one second (FEV1) of 2.50 liters (108% predicted) and a diffusing capacity of the lungs for carbon monoxide (DLCO) of 92% predicted. No lung quantitative scan was performed.
This case was discussed in multidisciplinary Tumor Board. Given the histologically confirmed malignancy in the lingula and highly suspicious left lower lobe mass, two treatment options were offered to the patient: (I) invasive mediastinal staging followed by intraoperative confirmation of synchronous primary tumors and left pneumonectomy or (II) left lower lobectomy with radiation to the lingular malignancy. After shared-decision making, our patient elected to undergo the option resulting in completion of left pneumonectomy.
Preoperative preparation
General anesthesia with selective single lung ventilation was performed using a double lumen endotracheal tube (18). After completion of bronchoscopy and a mediastinoscopy negative for abnormal mediastinal lymph nodes, our patient was positioned in the right lateral decubitus position with full hip flexion to maximize intercostal space (ICS) and to prevent impingement of the thoracoscope.
Equipment preference card
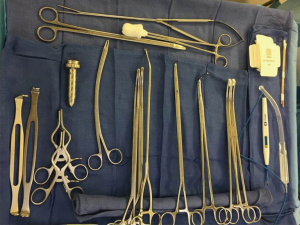
The surgical instrument tray contains the following equipment (Figure 4):
- 10 mm 30 degree rotating scope, 10mm short metal trocar;
- Long curved ring clamp;
- Short curved ring clamp;
- Harken (curved) clamps, long and short;
- Umbilical tapes;
- Long Metzenbaum scissors;
- DeBakey forceps;
- Weitlaner retractor;
- Low profile soft tissue retractor;
- Surgical stapler with tissue and vascular loads;
- Sponge stick with open thoracotomy tray (in the event of bleeding the sponge stick is used to tamponade the bleeding while the chest is opened);
- Surgical specimen bag.
Procedure
Port placement
Port placement is critical to successful VATS procedures to ensure correct video orientation. VATS procedures are complicated by the generation of paradoxical motion, produced when the camera and instruments are facing each other, however this can be amended by turning the camera 180 degrees to restore normal spatial orientation for the operator (18).
The first incision was created in the 9th ICS in the anterior axillary line for camera port access. Local anesthetic was injected into the ICSs to provide a nerve block that reduces surgical stimulation and eases postoperative pain. Once the pleural space was entered, the 12 mm metal trocar and 10 mm camera were introduced into the chest. In general, camera placement at the 8th or 9th ICS in the midaxillary line is optimal for right sided VATS, whereas for left sided procedures, posterior placement of the camera may be necessary to avoid visual obstruction by the pericardial fat pad (18). Additional incisions were made under direct vision in the 8th ICS in the posterior axillary line and the 6th ICS in the anterior axillary line for an access incision.
Initial inspection of the pleura, exposure of the hilum, and visualization of the inferior pulmonary ligament were accomplished via lung manipulation with ring forceps. Rib spreading was not necessary, but a low profile soft tissue retractor was used to separate the chest wall tissue, prevent lung expansion during suctioning and to provide adequate space for passage of instruments into the chest. Care was taken to avoid placing pressure on the inferior surface of the chest wall to prevent postoperative neuralgia from compression of intercostal nerves.
Release of the pulmonary ligament
Upon entry of the camera into the thorax, the thoracic cavity was inspected for evidence of pleural effusion and pleural studding. After adhesions between the lung and chest wall and mediastinum were separated, the inferior pulmonary ligament was dissected. The inferior pulmonary ligament was divided to the level of the inferior pulmonary vein and the posterior mediastinal pleura were opened to provide hilar mobilization. Inferior pulmonary ligament, periesophageal, and subcarinal lymph nodes were removed during this dissection. A wedge resection was performed to confirm malignancy in the left lower lobe mass.
Division of the inferior and superior pulmonary veins
Next, the inferior and superior pulmonary veins were dissected. Before division of the veins with a stapler, tension was released on the parenchyma to allow stapling via a tension-free technique. It may be necessary to also divide the anterior mediastinal pleura, thus facilitating mobilization of the veins off the pulmonary artery. All hilar nodes encountered were removed at this time.
Isolation and retraction of the bronchus
A combination of blunt and sharp dissection was used to isolate the bronchus and separate it from the left main pulmonary artery (LMPA). An umbilical tape was passed around the bronchus to allow for inferolateral retraction of the lung to facilitate exposure of the LMPA.
Division of the pulmonary artery and bronchus
Dissection and isolation of the pulmonary artery was achieved with combination blunt and sharp dissection and facilitated by removal of lymph nodes from stations 5, 6, 7 and 10. Approach from the anterior and posterior aspects of the hilum may be necessary to achieve a safe and complete dissection. The posterior pleura and other pleural attachments may also require dissection off the pulmonary artery and bronchus via sharp and blunt dissection. Inferolateral retraction of the bronchus is key during this portion of the procedure.
Once the pulmonary artery was isolated, a test occlusion was performed prior to division of the pulmonary artery with a stapler. In patients with questionable cardiac function or physiologic status, it may be best to approach pneumonectomy from a thoracotomy so that test occlusion of the pulmonary artery can be achieved before committing to pneumonectomy.
After division of the LMPA, the bronchus was the remaining intact structure and was divided with a stapler. Care should be afforded to stump length; if the tumor has endobronchial involvement, flexible bronchoscopy is used to verify exclusion of all tumor with the stapler. The lung was maximally retracted to allow for the shortest stump feasible. We placed the resected lung in a large specimen bag and it was removed from the patient through the access incision.
Systemic lymph node dissection
Mediastinal lymph node dissection was performed as is standard. Nodes were sampled from stations 5, 6, 7, 8, 9, and 10.
The thoracic cavity was irrigated and the bronchial stump was interrogated for air leak. Chest tube placement is at the surgeon’s discretion. In our case, we elected to place a tube to water seal that was removed on postoperative day one. Two nerve block catheters were placed at the 6th ICS and in the chest wall to provide continuous peripheral nerve block for postoperative pain management.
Role of team members
As with all surgical procedures, a “team” approach is necessary to facilitate efficiency in the operating theatre. The surgical team consists of:
- Surgeon and assistant;
- Anesthesiologist;
- Circulating nurse;
- Scrub nurse.
The surgeon is the leader and directs other team members to ensure coordination of care in the operating room. It is fundamental that the surgeon be trained in VATS technique and has experience with open pneumonectomy principles. Additionally, the surgeon should be prepared to guide the surgical team in addressing and resolving unanticipated intraoperative events and complications. Prior to initiation of this procedure, the surgeon should review emergency procedures audibly with the entire team. Blood availability should be discussed and technique for emergency thoracotomy should be addressed to ensure availability of correct surgical instruments.
The anesthesiologist is key member of the team responsible for monitoring and maintaining the patient’s hemodynamic and pulmonary stability. The anesthesiologist will perform single lung ventilation in the patient to avoid intraoperative hypoxemia. Additionally, the anesthesiologist must maintain analgesia throughout the operation to minimize surgical stimulation. Close communication regarding fluid management and blood pressure management, especially in preparation for division of the pulmonary artery is critical to the safety of VATS pneumonectomy.
Postoperative management
Postoperative course after pneumonectomy includes aggressive pulmonary and cardio-physiotherapy, strict aspiration precautions, and limitation of intravenous fluids (18).
Our patient was extubated in the operating room and transferred to the intensive care unit for close monitoring. Her chest tube was removed on postoperative day #1 and she was transferred to the general thoracic ward. Our patient’s pain was managed with intercostal nerve blockade, intravenous patient controlled analgesia (PCA) and oral analgesics. PCA was discontinued on postoperative day #0.
Careful selection of patients and detailed intraoperative management is key to the success of VATS pneumonectomy. Isolation of the bronchus from the pulmonary artery using intracorporeal retraction is key to the feasibility of this procedure. A cohesive team is necessary to ensure safety in the event of an intraoperative catastrophe. In select patients, VATS pneumonectomy offers a definitive therapeutic approach for synchronous primary malignancies.
Acknowledgements
Funding: The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent:Written informed consent was obtained from the patient for publication. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- Nakata M, Sawada S, Yamashita M, et al. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg 2004;78:1194-9. [Crossref] [PubMed]
- Beckles MA, Spiro SG, Colice GL, et al. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest 2003;123:105S-114S. [Crossref] [PubMed]
- Yim AP. VATS major pulmonary resection revisited--controversies, techniques, and results. Ann Thorac Surg 2002;74:615-23. [Crossref] [PubMed]
- Luo QQ, Lin H, Tan Q, et al. Analysis of clinical application of thoracoscopic lobectomy for lung cancer. World J Surg Oncol 2014;12:157. [Crossref] [PubMed]
- Piwkowski C, Gabryel P, Kasprzyk M, et al. Video-assisted thoracic surgery pneumonectomy: the first case report in Poland. Wideochir Inne Tech Maloinwazyjne 2012;7:197-201. [Crossref] [PubMed]
- Battafarano RJ, Meyers BF, Guthrie TJ, et al. Surgical resection of multifocal non-small cell lung cancer is associated with prolonged survival. Ann Thorac Surg 2002;74:988-93; discussion 993-4. [Crossref] [PubMed]
- Nwogu CE, Glinianski M, Demmy TL. Minimally invasive pneumonectomy. Ann Thorac Surg 2006;82:e3-4. [Crossref] [PubMed]
- Kaseda S, Aoki T, Hangai N, Shimizu K. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg 2000;70:1644-6. [Crossref] [PubMed]
- Augustin F, Maier H, Lucciarini P, et al. Extended minimally invasive lung resections: VATS bilobectomy, bronchoplasty, and pneumonectomy. Langenbecks Arch Surg 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Demmy TL, Curtis JJ. Minimally invasive lobectomy directed toward frail and high-risk patients: a case-control study. Ann Thorac Surg 1999;68:194-200. [Crossref] [PubMed]
- Chen HW, Du M. Video-assisted thoracoscopic pneumonectomy. J Thorac Dis 2015;7:764-6. [PubMed]
- Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8. [Crossref] [PubMed]
- Miller D, Will M, Walker W. VATS Pneumonectomy: The Posterior Approach. Journal of Cardiothoracic Surgery 2015;10:A350. [Crossref]
- Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg 2014;148:637-43. [Crossref] [PubMed]
- Phillips JD, Merkow RP, Sherman KL, et al. Factors affecting selection of operative approach and subsequent short-term outcomes after anatomic resection for lung cancer. J Am Coll Surg 2012;215:206-15. [Crossref] [PubMed]
- Tsubota N. Is pneumonectomy using video-assisted thoracic surgery the way to go? Study of data from the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2014;62:499-502. [Crossref] [PubMed]
- Kaiser LR, Kron IL, Spray TL, editors. Mastery of Cardiothoracic Surgery. 2 ed. Philadelphia: Lippincott Williams & Wilkins, 2007.
- Yu WS, Lee CY, Lee S, et al. Trainees Can Safely Learn Video-Assisted Thoracic Surgery Lobectomy despite Limited Experience in Open Lobectomy. Korean J Thorac Cardiovasc Surg 2015;48:105-11. [Crossref] [PubMed]
- Nwogu CE, Yendamuri S, Demmy TL. Does thoracoscopic pneumonectomy for lung cancer affect survival? Ann Thorac Surg 2010;89:S2102-6. [Crossref] [PubMed]
- Li F, Zhong WZ, Niu FY, et al. Multiple primary malignancies involving lung cancer. BMC Cancer 2015;15:696. [Crossref] [PubMed]
- Xue X, Liu Y, Pan L, et al. Diagnosis of multiple primary lung cancer: a systematic review. J Int Med Res 2013;41:1779-87. [Crossref] [PubMed]
- Hashimi H, Cooke DT, David EA, et al. Video-assisted thoracoscopic surgery (VATS) left pneumonectomy for synchronous primary lung malignancies. Asvide 2016;3:186. Available online: http://www.asvide.com/articles/942
- Anagnostou VK, Syrigos KN, Bepler G, et al. Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. J Clin Oncol 2009;27:271-8. [Crossref] [PubMed]
Cite this article as: Hashimi H, Cooke DT, Holmes SK, Brown LM, David EA. Video-assisted thoracoscopic surgery: pneumonectomy for synchronous primary lung malignancies. J Vis Surg 2016;2:67.