
Thoracoscopic laser pulmonary metastasectomy
Introduction
Pulmonary metastasectomy is a commonly performed thoracic surgical procedure with approximately 30% of all cancer patients developing lung metastases at some point (1).
Pulmonary metastasectomy has evolved since its conception in 1930. Traditionally these procedures were performed through a full thoracotomy or sternotomy (2,3). The surgical approaches to metastasectomy have evolved alongside other thoracic procedures with the advent of new technologies and the demand for minimally invasive techniques. Nd:YAG lasers have been used for pulmonary metastasectomy via thoracotomy, proving to be a safe and oncologic technique (3,4). In recent years, pulmonary metastasectomy using the Nd:YAG laser has evolved to encompass a thoracoscopic approach with good results. We discuss this approach and the surrounding technical considerations.
Indications
The currently accepted indications for pulmonary metastasectomy are:
- The primary malignancy must be controlled or controllable.
- There must be an absence of extra-thoracic metastasis or resectable extra-thoracic metastasis.
- The tumour must be fully resectable with adequate remaining pulmonary reserve
- There must be an absence of medical treatment options with lower morbidity (3,5-7).
Surgical approach
The surgical approach for thoracoscopic laser metastasectomy follows conventional two or three port thoracoscopic technique. Single lung ventilation is required either using a bronchial blocker or a double lumen endotracheal tube. A 2–5 cm anterior utility incision in the 4th or 5th intercostal space is typically used as the main working port. A wound protection device such as the Alexis Protector (Applied Medical) can be used in this incision to aid with retraction (8,9). This port is used for finger palpation of the lung parenchyma for localisation of the lesion. The hand-held laser device is then introduced through this port. In addition this port provides access for smoke extraction. A standard inferior 5–10 mm camera port is used. A third incision can be created to allow for control of the lung aiding in bimanual palpation of the lung parenchyma through the utility port.
Once the lesion is satisfactorily located, the lung is grasp to position the lesion in a safe and accessible position for resection. The laser beam is directed onto the lung tissue with the aid of a red pilot light, which is transmitted down the delivery fibre. The red dot of the pilot light can be seen in Figure 1. The laser is activated using a foot pedal.
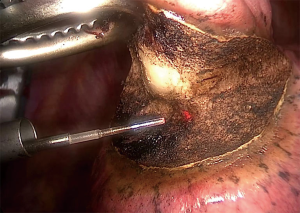
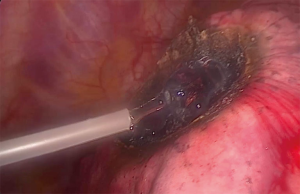
Figure 2 demonstrates the dissection capabilities of the KLS Martin Limax Nd:YAG laser at 1.318 µm. Once the lesion has been fully resected the resection bed is coagulated to ensure haemostasis and pneumostasis. A haemostatic agent can be applied to the area as in Figure 3. A single chest drain is inserted at the end of the procedure.

Technical considerations
The laser
On contact with the lung parenchyma the lasers light energy is converted into extreme heat with temperatures of over 900 °C. This vaporises the tissue creating the accurate cutting action that is required. The tip of the delivery fibre is positioned between 1 to 30 mm from the target tissue. The closer the tip of the fibre is to the tissue the more effective the cutting versus coagulation. The coagulation of the resection surface ensures a largely bloodless resection field throughout (8,9).
Wire guidance
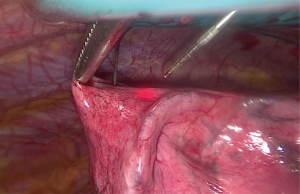
Pre-operative imaging can determine the degree of accessibility of the target lesions during a VATS resection. Smaller lesions or those deep within the lung parenchyma can be difficult to identify with sufficient confidence intra-operatively. Previously with thoracoscopic resection, intra-operative failure to identify the target lesion was a reason for conversion to open (11). CT guided wire localisation can be used to accurately demonstrate the target area. The use of wire guidance is a safe and cost effective method of identifying the target lesion (12). A hooked wire is placed under CT guidance, circumferential resection of the lesion can be safely performed with the wire remaining in situ. This can be seen in Figure 4.
Smoke extraction
Vaporisation of the lung parenchyma with the LIMAX Nd:YAG 1.318 µm Laser results in the creation of significant volumes of smoke. Without adequate air extraction systems this can quickly obliterate the thoracoscopic view making safe dissection impossible. The laser device itself is fitted with an inbuilt suction system for smoke extraction. The addition of supplementary smoke evacuator tubing held at the external aspect of the utility port improves smoke extraction from within the chest cavity. This aids in an unobstructed view allowing safer and faster resections (8).
The use of haemostatic agents
The use of a haemostatic agent in the prevention of pulmonary airleak has been described and is shown in Figure 2. In all patients where PuraStat® was applied directly to the resected surfaces following thoracoscopic laser metastasectomy no air-leak occurred (8). The prevention of air leak is paramount in achieving early drain removal and reducing post-operative length of stay. Prolonged air-leak increases both length of stay and increases hospital costs (13). Sutures have also been used in addition to laser coagulation. When completing deeper resections with the laser (>0.5 cm), Meyer et al. closed the tissue with an overlock stitch using endoscopic needle holders (9).
Comparison with stapling devices
The Nd:YAG 1.318 µm laser has been shown to be a safe and efficient method for performing oncologic pulmonary resections. Using the laser for pulmonary resection has a number of additional benefits. When stapled resection is used radiological follow-up can be difficult and it can be impossible to differentiate local reaction to the staples versus local recurrence. With the laser, no foreign material remains within the lung parenchyma facilitating easier and more accurate interpretation of follow-up imaging (8). Stapling devices are known to cause dense adhesions within the thoracic cavity. This can make any attempted redo surgery difficult. During redo metastastectomy any lesion near the previous stapled tissue becomes hard to localise intra-operatively. As mentioned previously the failure to adequately localise the lesion may result in conversion to thoracotomy with its associated patient complications. The use of stapling devices results in more loss of normal lung parenchyma due to the straight lines and the limited permitted geometry of the stapler. The tissue loss that occurs with laser resection is in the order of one seventh of that occurring for staple resections independent of the location of the metastasis (4).
Radical and multiple resections
Given the lung sparing capabilities of the laser, radical metastasectomies can be undertaken for lesions in multiple lobes that would not otherwise be feasible. This includes deep parenchymal lesions (3).
Cost
The regular use of the laser has been shown to be cost effective when compared to the equivalent operations performed using stapling devices. The initial fixed cost of the laser was recovered following the first 300 cases performed without staples (14).
Postoperative management
The post-operative management of these patients mirrors that of other thoracoscopic techniques. Optimal analgesic control is important as with all thoracic surgery. A pleural drain is required to monitor for air leak and facilitate lung re-expansion. Mean time to drain removal is reported at 1–4 days (8,9). The use of the Thopaz digital chest drain (Medela) system allows continuous objective monitoring for air leak, facilitating early removal of the drain. This subsequently facilitates rapid post-operative recovery and discharge. The post-operative length of stay for these procedures is short. Median lengths of hospital stay reported are reported at 1 and 5 days (8,9).
Our experience
To date, in our centre, seven patients have undergone thoracoscopic laser wedge metastasectomy of 8 lesions. All patients met the criteria for pulmonary metastasectomy in that the primary disease had been controlled, they did not have any distant metastatic disease and the lesion was amenable to wedge resection on CT with sufficient remaining pulmonary reserve. There were no technical complications with the laser device during any of the cases. No cases required conversion to a full thoracotomy. The drain was removed on post-operative day 1 in 6 cases and on day 8 in the remaining case. The median post-operative LOS was 1 day (range: 1–10 days), SD 4.36. All patients had confirmed metastatic disease with clear resection margins on final histopathology. There were no peri-operative mortalities. One patient had a prolonged air leak requiring the drain to remain in situ until post-operative day 8.
In our early experience, thoracoscopic laser wedge metastasectomy is a safe and efficient method for performance of pulmonary metastasectomy. We experienced a low complication rate and a short post-operative stay (8). The concept of thoracoscopic laser resection is currently being expanded into our other areas of practice including lung biopsies, the resection of benign lesions and the division of lung fissures.
Conclusions
The use of an 1.318 µm Nd:YAG laser has been shown to be an effective approach for pulmonary metastasectomy during open surgery. Its usage is gaining popularity in the setting of minimally invasive surgery. This technique provides the proven benefits of minimally invasive surgery combined with the benefits of laser resection compared to conventional stapled wedge resections. Overall it is a safe and oncological surgical technique with many benefits to the patient and a typically short post-operative length of stay. This technique is like to gain further popularity for non-anatomical pulmonary resections.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michel Gonzalez) for the series “Advancement in the Surgical Treatment of Pulmonary Metastasis” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.06.03). The series “Advancement in the Surgical Treatment of Pulmonary Metastasis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davidson RS, Nwogu CE, Brentjens MJ, et al. The surgical management of pulmonary metastasis: current concepts. Surg Oncol 2001;10:35-42. [Crossref] [PubMed]
- Barney JD, Churchill EJ. Adenocarcinoma of the kidney with metastasis to the lung: cured by nephrectomy and lobectomy. J Urol 1939;42:269-76. [Crossref]
- Osei-Agyemang T, Palade E, Haderthauer J, et al. Pulmonary metastasectomy: an analysis of technical and oncological outcomes in 301 patients with a focus on laser resection. Zentralbl Chir 2013;138:S45-51. [PubMed]
- Rolle A, Pereszlenyi A, Koch R, et al. Laser resection technique and results of multiple lung metastasectomies using a new 1,318 nm Nd:YAG laser system. Lasers Surg Med 2006;38:26-32. [Crossref] [PubMed]
- Erhunmwunsee L, Tong BC. Preoperative evaluation and indications for pulmonary metastasectomy. Thorac Surg Clin 2016;26:7-12. [Crossref] [PubMed]
- Jaklitsch MT, Mery CM, Lukanich JM, et al. Sequential thoracic metastasectomy prolongs survival by re-establishing local control within the chest. J Thorac Cardiovasc Surg 2001;121:657-67. [Crossref] [PubMed]
- Kondo H, Okumura T, Ohde Y, et al. Surgical treatment for metastatic malignancies. Pulmonary metastasis: indications and outcomes. Int J Clin Oncol 2005;10:81-5. [Crossref] [PubMed]
- Mc Loughlin JB, O’Sullivan KE, Brown R, et al. Limax Nd:YAG laser-assisted thoracoscopic resection of pulmonary metastases; a single centre’s initial experience. Ir J Med Sci 2019;188:771-6. [PubMed]
- Meyer C, Bartsch D, Mirow N, et al. Video-assisted laser resection of lung metastases-feasibility of a new surgical technique. Thorac Cardiovasc Surg 2017;65:382-6. [Crossref] [PubMed]
- Mc Loughlin JB, O’Sullivan KE, Brown RH, et al. A 70 second video demonstrating the laser resection performed in Figure 1 including addition of Purastat. Asvide 2019;6:210. Available online: http://www.asvide.com/watch/32888
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Rathinam S, Nakas A, Bajaj A. A simple and safe technique for CT guided lung nodule marking prior to video assisted thoracoscopic surgical resection revisited. Lung Cancer Int 2015;2015:235720 [PubMed]
- Varela G, Jimenez MF, Novoa N, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329-33. [Crossref] [PubMed]
- Marulli G, Droghetti A, Di Chiara F, et al. A prospective randomized trial comparing stapler and laser techniques for interlobar fissure completion during pulmonary lobectomy. Lasers Med Sci 2013;28:505-11. [Crossref] [PubMed]
Cite this article as: Mc Loughlin JB, O’Sullivan KE, Brown RH, Eaton DA. Thoracoscopic laser pulmonary metastasectomy. J Vis Surg 2019;5:64.


