Surgical treatment of synchronous multiple lung adenocarcinomas: experiment of 171 cases
Introduction
Synchronous multiple lung adenocarcinoma (SMLA) cases are increasing with the development of high-resolution computed tomography (HRCT). In these cases, multiple ground glass opacity nodules (GGN) are detected in the peripheral lung, and are often diagnosed as adenocarcinomas with lepidic growth or atypical adenomatous hyperplasia (AAH). The prognoses are reported to be relatively good (1,2). In these cases, surgeons tend to choose lung-preserving surgery because of the multiple lesions in the peripheral lung, which are often bilateral. Limited resection for single GGN is reported to be acceptable in some non-randomized trials (3,4), and clinical trials are ongoing in Japan (5,6). The National Comprehensive Cancer Network (NCCN) guideline recommends clinical follow-up for pure GGN less than 2 cm (7).
In this study, the prognosis of SMLA after surgery was retrospectively reviewed, and optimal treatment was examined.
Methods
We included 171 patients with SMLA who underwent surgical resection between January 2010 and March 2018 in Kumamoto University Hospital. SMLAs were diagnosed clinically, at least two nodules were noted on HRCT, and the nodules were pathologically diagnosed as lung adenocarcinomas; pulmonary metastases were deniable based on CT images or pathological findings. Mucinous adenocarcinomas, which show a pneumonia-like shadow in the peripheral lung, were excluded from this study. Our policies of treatment for SMLA were as follows: (I) treatments for the most progressive nodules were prioritized; (II) lung volume-preserving surgery, segmentectomy or wedge resection, was preferred for GGN (part solid nodules or pure GGN) within 2.0 cm; (III) pure GGN were resected by wedge resection, if possible. Lipiodol markings were performed for untouchable nodules; and (IV) clinical observation was allowed for pure GGN within 1.0 cm. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by ethical committee of Kumamoto University (No. 202, 402). Written informed consent was obtained from all patients.
Seventy-one patients were male, and 100 were female. Their median [range] age was 70 [39–87] years. Smoking histories were negative in 99 patients, and positive in 72. The numbers of nodules first found on HRCT were 2 in 86 patients, 3 in 32 patients, 4 in 35 patients and more than 4 in 18 patients. Pathological stages of the most progressive nodule were 0/IA1 in 101 cases (59.1%), IA2/IA3 in 49 cases (28.7%) and IB or above (IB in 11, IIA in 2, IIB in 5, IIIA in 2 and IIIB in 1) in 21 cases (12.3%). The most surgeries performed in each lung are shown in Table 1. Bilateral surgeries were performed in 19 patients. In 49 cases, limited resection (segmentectomy or wedge resection) was performed for the most progressive nodules. A representative case of surgery for SMLA is shown in Video 1. Right S3 segmentectomy for a part-solid nodule and rt. S6 wedge resection for a pure GGN nodule were performed. A pure GGN nodule in the left upper lobe was left unresected and observed clinically.
Table 1
| Variable | Surgical procedure | N |
|---|---|---|
| Bilateral | Lobectomy + lobectomy | 5 |
| Bilobectomy + segmentectomy | 1 | |
| Lobectomy + segmentectomy | 2 | |
| Lobectomy + partial | 8 | |
| Segmentectomy + partial resection | 3 | |
| Ipsilateral | Pneumonectomy | 2 |
| Bilobectomy | 4 | |
| Lobectomy | 100 | |
| Segmentectomy | 17 | |
| Partial resection | 29 |
SMLA, synchronous multiple lung adenocarcinoma.
Statistical methodology
All statistical analyses were performed with the SPSS program for Windows (version 18 statistical software; Texas Instruments, IL, USA). Numerical data were compared with an independent sample t-test, and categorical data were compared with a Pearson chi-square test. Recurrence-free survival (RFS) and overall survival (OS) were calculated from the date of surgery and estimated using Kaplan-Meier analysis. Differences between curves were evaluated using log-rank tests. To evaluate whether a biomarker was an independent prognostic factor for survival, a multivariate, stage-stratified Cox proportional hazard model was constructed to compute a hazard ratio (HR) that was based on the clinicopathological parameters. All p values were two-sided, and differences with P values of less than 0.05 were considered significant.
Results
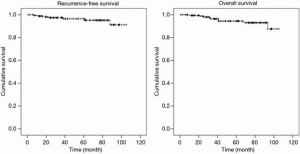
Kaplan-Meier curves for all patients are shown in Figure 1. The median follow-up period was 51.8 months. The 5-year RFS rate after surgery was 95.2%, and the OS rate was 93%.
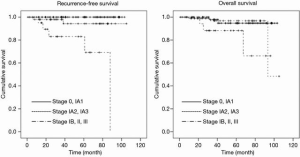
Survival comparisons among pathological stages of the dominant tumor are shown in Figure 2. The 5-year RFS rate was 100% for stage 0/IA1 and 94.3% for stage IA2/IA3; these rates were significantly higher than for stage IB and above (83.0%: P<0.01). The 5-year OS rate was 94.7% for stage 0/IA1 and 96.8% for stage IA2/IA3; these rates were significantly higher than for stage IB and above (88.2%: P=0.05). With regard to RFS, the survival analysis for clinicopathological factors is shown in Table 2. A CT finding of solid, high carcinoembryonic antigen (CEA) level (>5.0 µg/L), pleural invasion, vascular invasion, and lymph node metastasis were significantly associated with poor prognosis on univariate analysis. However, only a CT finding of solid remained significant on multivariate analysis. As for OS (Table 3), high age (>75 years) and high CEA (>5.0 µg/L) were significantly associated with poor prognosis. Both remained significant on multivariate analysis. Tumor number (>3), surgical procedure (limited surgery) or remnant tumors were not associated with prognosis.
Table 2
| Variable | N | HR | 95% CI | P value | P value (multivariate) |
|---|---|---|---|---|---|
| Gender | N.S. | – | |||
| Female | 100 | 1 | – | ||
| Male | 71 | 0.98 | 0.23–4.58 | ||
| Age, years | N.S. | – | |||
| ≤75 | 122 | 1 | – | ||
| >75 | 49 | 0.47 | 0.06–3.90 | ||
| Smoking status | N.S. | – | |||
| Negative | 99 | 1 | – | ||
| Positive | 72 | 1.31 | 0.29–5.89 | ||
| CT finding | 0.005 | 0.048 | |||
| GGN | 122 | 1 | – | ||
| Solid | 49 | 21.40 | 2.52–181.06 | ||
| Tumor number | N.S. | – | |||
| ≤3 | 118 | 1 | – | ||
| >3 | 53 | 1.70 | 0.37–7.60 | ||
| CEA, μg/L | 0.046 | N.S. | |||
| ≤5.0 | 153 | 1 | – | ||
| >5.0 | 18 | 5.95 | 1.07–33.07 | ||
| Surgery | N.S. | – | |||
| S or P | 49 | 1 | – | ||
| Lobectomy | 122 | 2.93 | 0.35–34.64 | ||
| Pleural invasion | 0.001 | N.S. | |||
| Negative | 159 | 1 | – | ||
| Positive | 12 | 12.33 | 2.74–55.47 | ||
| Vascular invasion | 0.001 | N.S. | |||
| Negative | 162 | 1 | – | ||
| Positive | 9 | 13.94 | 3.11–60.45 | ||
| LN metastasis | <0.001 | N.S. | |||
| Negative | 164 | 1 | – | ||
| Positive | 7 | 24.00 | 5.27–109.26 | ||
| Remnant tumor | N.S. | – | |||
| Negative | 124 | 1 | – | ||
| Positive | 47 | 0.45 | 0.05–3.76 |
HR, hazard ratio; CI, confidence interval; N.S., not significant; GGN, ground glass opacity nodules; CEA, carcinoembryonic antigen; S, segmentectomy; P, partial resection; LN, lymph node.
Table 3
| Variable | N | HR | 95% CI | P value | P value (multivariate) |
|---|---|---|---|---|---|
| Gender | N.S. | – | |||
| Female | 100 | 1 | – | ||
| Male | 71 | 2.59 | 0.65–10.37 | ||
| Age, years | 0.001 | 0.008 | |||
| ≤75 | 122 | 1 | – | ||
| >75 | 49 | 10.33 | 2.10–50.91 | ||
| Smoking status | N.S. | – | |||
| Negative | 99 | 1 | – | ||
| Positive | 72 | 3.60 | 0.89–14.50 | ||
| CT finding | N.S. | – | |||
| GGN | 122 | 1 | – | ||
| Solid | 49 | 2.68 | 0.71–10.11 | ||
| Tumor number | N.S. | – | |||
| ≤3 | 118 | 1 | – | ||
| >3 | 53 | 0.32 | 0.04–2.55 | ||
| CEA, μg/L | 0.046 | 0.03 | |||
| ≤5.0 | 153 | 1 | – | ||
| >5.0 | 18 | 7.15 | 1.69–30.32 | ||
| Surgery | N.S. | – | |||
| S or P | 49 | 1 | – | ||
| Lobectomy | 122 | 1.69 | 0.35–8.23 | ||
| Pleural invasion | N.S. | – | |||
| Negative | 159 | 1 | – | ||
| Positive | 12 | 2.11 | 0.26–17.0 | ||
| Vascular invasion | N.S. | – | |||
| Negative | 162 | 1 | – | ||
| Positive | 9 | 2.33 | 0.19–18.91 | ||
| LN metastasis | N.S. | – | |||
| Negative | 164 | 1 | – | ||
| Positive | 7 | 4.81 | 0.59–39.39 | ||
| Remnant tumor | N.S. | – | |||
| Negative | 124 | 1 | – | ||
| Positive | 47 | 1.55 | 0.39–6.10 |
HR, hazard ratio; CI, confidence interval; N.S., not significant; GGN, ground glass opacity nodules; CEA, carcinoembryonic antigen; S, segmentectomy; P, partial resection; LN, lymph node.
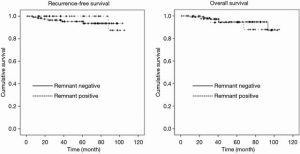
Differences between the groups with and without remnant tumors are shown in Table 4. There was no significant difference between the groups except in tumor number (P=0.03). Existence of remnant tumors was not associated with prognosis for either RFS or OS (Figure 3).
Table 4
| Variables | Remnant negative | Remnant positive | P value |
|---|---|---|---|
| Gender | N.S. | ||
| Male | 55 | 16 | |
| Female | 69 | 31 | |
| Age (mean ± SD) | 68.4±9.4 | 68.6±8.9 | N.S. |
| Smoking history | N.S. | ||
| Positive | 69 | 30 | |
| Negative | 55 | 17 | |
| Tumor number (CT) | 0.03 | ||
| 2 | 71 | 15 | |
| 3 | 23 | 9 | |
| ≥4 | 30 | 23 | |
| CT findings of dominant tumor | N.S. | ||
| GGN | 91 | 31 | |
| Solid | 33 | 16 | |
| pStage of dominant tumor | N.S. | ||
| 0/IA1 | 75 | 26 | |
| IA2/IA3 | 32 | 17 | |
| Above IB | 17 | 4 |
N.S., not significant; SD, standard deviation; GGN, ground glass opacity nodules.
Discussion
Lepidic-type adenocarcinoma is known to occur frequently in Asians and non-smokers. The prognosis is good, and the propensity of lymph node and distant metastasis is low in comparison to solid adenocarcinomas (9). Multifocal parenchymal nodules are frequently found in patients with lepidic-type adenocarcinoma (10). In this study, we retrospectively reviewed the results of surgery for SMLAs. The most progressive nodules of our cases were GGN (part solid nodules or pure GGN) in 122 cases (71.3%), and their pathological stages were stage 0/IA in 101 cases (59.1%). That is why the prognosis of our series was extremely good (5-year RFS was 95.2%, and OS was 93%).
In the treatment of SMLAs, surgeons are forced to select limited resection to preserve lung function. The standard surgical procedure for lung cancer, even for small-sized lung cancer, is lobectomy with hilar and mediastinal nodal dissection, based on the results of a randomized controlled trial (11). Based on the results of a prospective multi-institutional study on the relationship between radiologic and pathologic findings in peripheral lung cancer (12), the Japan Clinical Oncology Group (JCOG) and the West Japan Oncology Group (WJOG) conducted a randomized, phase III trial investigating lobectomy versus limited resection for small invasive peripheral lung cancers (6). In our series, limited resection for dominant tumors was performed in 28.6% of patients, and surgical procedure was not associated with prognosis. In addition, the non-dominant tumors, mostly GGN, were frequently treated with limited resection. However, the survival of our patients was extremely good.
According to several studies (13,14), solitary pure GGN should be followed up until they increase in size or develop a new solid component. Nevertheless, whether this strategy is also appropriate for SMLAs is controversial. In this study, although GGN were left in 27.5% of cases, this was not associated with prognosis. In some cases, we could not treat all of the nodules because there were multifocal nodules in the lungs bilaterally. Our strategy for SMLA cases was to focus on invasive nodules, with CT findings that were solid or sub-solid, and to leave pure GGN unresected and observe them clinically. When a GGN became enlarged or a solid component appeared, additional local treatment, including radiofrequency ablation or stereotactic radiation therapy, could be incorporated.
This study had some limitations. Due to its retrospective nature, a prospective study is necessary to verify the results. The observation period was relatively short, and the safety of clinical observation of remnant tumors is unclear.
In conclusion, the prognosis of SMLA in these patients was relatively good. The prognosis was dependent on the pathological stage of the dominant tumor. Non-solid tumors less than 1 cm can be clinically observed, and GGN can be treated with limited surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Meinoshin Okumura) for the series “Dedicated to the 36th Annual Conference of Japanese Association for Chest Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs.2019.12.07/coif). The series “Dedicated to the 36th Annual Conference of Japanese Association for Chest Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by ethical committee of Kumamoto University (No. 202, 402). Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gao RW, Berry MF, Kunder CA, et al. Survival and risk factors for progression after resection of the dominant tumor in multifocal, lepidic-type pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2017;154:2092-2099.e2. [Crossref] [PubMed]
- Liu M, He W, Yang J, et al. Surgical treatment of synchronous multiple primary lung cancers: a retrospective analysis of 122 patients. J Thorac Dis 2016;8:1197-204. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Sublobar resection for lung adenocarcinoma meeting node-negative criteria on preoperative imaging. Ann Thorac Surg 2014;97:1701-7. [Crossref] [PubMed]
- Okumura M, Goto M, Ideguchi K, et al. Factors associated with outcome of segmentectomy for non-small cell lung cancer: long-term follow-up study at a single institution in Japan. Lung Cancer 2007;58:231-7. [Crossref] [PubMed]
- Aokage K, Saji H, Suzuki K, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg 2017;65:267-72. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:412-41. [Crossref] [PubMed]
- Ikeda K, Fujino K, Masuda Y, et al. A representative case of surgery for SMLA. Asvide 2020;7:034. Available online: http://www.asvide.com/watch/33074
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Roberts PF, Straznicka M, Lara PN, et al. Resection of multifocal non-small cell lung cancer when the bronchioloalveolar subtype is involved. J Thorac Cardiovasc Surg 2003;126:1597-602. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol 2011;6:751-6. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the Management of Subsolid Pulmonary Nodules Detected at CT: A Statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Godoy MC, Naidich DP. Overview and strategic management of subsolid pulmonary nodules. J Thorac Imaging 2012;27:240-8. [Crossref] [PubMed]
Cite this article as: Ikeda K, Fujino K, Masuda Y, Suzuki M. Surgical treatment of synchronous multiple lung adenocarcinomas: experiment of 171 cases. J Vis Surg 2020;6:15.